Abstract
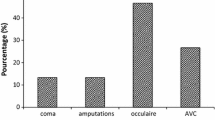
The formation of Advanced Glycation Endproducts (AGEs) has been found to play a role in the development of diabetic symptoms. Production of methylglyoxal (MG), a highly cytotoxic and crosslinking aldehyde, is elevated among patients with type 2 diabetes mellitus (T2DM) and is a precursor to AGEs. The ubiquitous glyoxalase system is one of several defense mechanisms involved in MG metabolism and the protection against the production of AGEs. The system is a complex of two enzymes: glyoxalase I (GloI) that converts MG and reduced glutathione (GSH) to S-lactoylglutathione which is converted to D-lactic acid by glyoxalase II, regenerating GSH in the process. The malfunctioning of the glyoxalase system results in the accumulation of MG. The present study was performed to explore the relationship between the decreased activity of GloI and the complications associated to T2DM. The activities of the GloI, GloII and the concentration of GSH were measured in blood samples of 203 volunteers: 75 controls, 60 non-insulino-dependent diabetes mellitus (NIDDM) individuals and 68 NIDDM patients with complications as follow: 18 with nephropathy, 15 with retinopathy, 15 with neuropathy and 20 with macroangiopathy. All individuals were from the northen region of Morocco. We also evaluated the relationships between GloI levels and the pathogenesis of micro- and macrovascular complications of diabetes. We found a significant decrease in the GloI activity and GSH levels in patients with diabetes compared to controls. GloI activity was further reduced in samples from diabetes patients with complications. The levels of GloI were markedly lower in blood samples from patients with nephropathy than in uncomplicated patients and normal subjects. In contrast, there was no significant change in the activity of GloII in NIDDM patients compared to controls. This study suggests that the low level of GloI activity in T2DM patients is most probably due to decreased level of GSH content and reflects the role of GloI enzyme in protecting T2DM patients from AGEs accumulation and further complications.
Similar content being viewed by others
References
Kahn CR. Banting lecture: insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43:1066–84.
Hamman RF. Genetic and environmental determinants of non-insulin-dependent diabetes mellitus (NIDDM). Diabetes Metab Rev. 1992;8:287–338.
Betz AL, Bowman PD, Goldstein GW. Hexose transport in microvascular endothelial cells cultured from bovine retina. Exp Eye Res. 1983;36:269–77.
Keen KM, Jarrett RJ. Complications of diabetes. 2nd ed. London: Edward Arnold Publishers; 1982. p. 1–270.
Brownlee M. Glycation and diabetic complications. Diabetes. 1994;43:836–41.
Vander Jagt DL, Robinson B, Taylor KK, Hunsaker LA. Reduction of trioses by NADPH-dependent aldo-keto reductases: aldose reductase, methylglyoxal, and diabetic complications. J Biol Chem. 1992;267:4364–9.
Szwergold BS, Kappler F, Brown TR. Identification of fructose 3-phosphate in the lens of diabetic rats. Science. 1990;247:451–4.
Baskaran S, Balasubramanian KA. Effect of methylglyoxal on protein thiol and amino groups in isolated rat enterocytes and colonocytes and activity of various brush border enzymes. Indian J Biochem Biophys. 1990;27(1):13–7.
Lal S, Szwergold BS, Taylor AH, Randall WC, Kappler F, Wells-Knecht K, et al. Metabolism of fructose-3.phosphate in the diabetic rat lens. Arch Biochem Biophys. 1995;318:191–9.
Knecht K, Feather M, Baynes J. Detection of 3-deoxyfructose and 3-deoxyglucosone in human urine and plasma: evidence for intermediate stages of the Maillard reaction in vivo. Arch Biochem Biophys. 1992;294:130–7.
Beisswenger P, Lal S, Howell S, Stevens R, Siegel A, Yeo K, et al. The role of 3-deoxyglucosone and the activity of its degradative pathways in the etiology of diabetic microvascular disease. In: O’Brien J, Nursten HE, Crabbe MJC, Ame JM, editors. The Maillard reaction in foods and medicine. U.K: Royal Society of Chemistry; 1998. p. 298–303.
Beisswenger PJ, Moore LL, Truls BJ, Curphey TJ. Increased collagen-linked pentosidine and advanced glycosylation end products in early diabetic nephropathy. J Clin Invest. 1993;92:212–7.
Monnier V, Vishwanath V, Frank KF, Elmets CAK, Sauthot P, Kohn RR. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986;314:403–8.
Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269:1–11.
Ratliff DM, Vander Jagt DJ, Eaton RP, Vander Jagt DL. Increased levels of methylglyoxal-metabolizing enzymes in mononuclear and polymorphonuclear cells from insulin-dependent diabetic patients with diabetic complications: aldose reductase, glyoxalase I and glyoxalase II—A clinical research center study. J Clin Endocrinol Metab. 1996;81:488–92.
Vander Jagt DL, Hunsaker LA. Methylglyoxal metabolism and diabetic complications: roles of aldose reductase, glyoxalase I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chem Biol Interact. 2003;143–144:341–51.
Vander Jagt DL. The glyoxalase system. In: Dolphin D, Paulson R, Avramovic 0; editors. Glutathione: chemical, biochemical, and medical aspects. Wiley and Sons, Part A; 1989; 598–641.
Creighton DJ, Hamilton DS. Brief history of glyoxalase I and what we have learned about metal ion-dependent, enzyme-catalyzed isomerizations. Arch Biochem Biophys. 2001;387:1–10.
Vander Jagt DL, Hassebrook RK, Hunsaker LA, Brown WM, Royer RE. Metabolism of the 2-oxoaldehyde methylglyoxal by aldose reductase and by glyoxalase- I: roles for glutathione in both enzymes and implications for diabetic complications. Chem Biol Interact. 2001;130–132:549–62.
Vander Jagt DL, Han LPB, Lehman CH. Kinetic evaluation of substrate specificity in the glyoxalase-I-catalyzed disproportionation of α-ketoaldehydes. Biochemistry. 1972;11(20):3735–40.
Uotila L. Preparation and assay of glutathione thiol esters. Survey of human liver gluta- thione thiol esterases. Biochemistry. 1973;12:3938–47.
Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–55.
Thornalley PJ. Glutathione-dependent detoxification of a-oxoaldehydes by the glyoxalase system: involvement in disease mechanisms and antiproliferative activity of glyoxalase I inhibitors. Chem Biol Interact. 1998;111–112:137–51.
Miyata T, Ypersele De Strihou CV, Imasawa T, Yoshino A, Ueda Y, Ogura H, et al. Glyoxalase I deficiency is associated with an unusual level of advanced glycation end products in a hemodialysis patient. Kidney Int. 2001;60:2351–9.
Penninckx MJ, Jaspers CJ, Legrain MJ. The glutathione dependent glyoxalase pathway in yeast Saccharomyces cerevisiae. A vital defence line against methylglyoxal producing during glycerol catabolism. J Biol Chem. 1983;258:6030–8.
Cooper RA, Anderson A. The formation and catabolism of methylglyoxal during glycolysis in Escherichia coli. FEBS Lett. 1970;11:273–6.
Kalapos MP. Methylglyoxal toxicity in mammals. Toxicol Lett. 1994;73:3–24.
Ankrah NA, Appiah-Opong R. Toxicity of low levels of methylglyoxal: depletion of blood glutathione and adverse effect on glucose tolerance in mice. Toxicol Lett. 1999;109:61–7.
Shamsi FA, Sharkey E, Creighton D, Nagaraj RH. Maillard reactions in lens proteins: methylglyoxal mediated modifications in the rat lens. Exp Eye Res. 2000;70:369–80.
Thornalley PJ. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification—a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–73.
Ray S, Ray M. Formation of methylglyoxal from aminoacetone by amine oxidase from goat plasma. J Biol Chem. 1983;258:3461–2.
Reichard GA, Skutches CL, Hoeldtke RD, Owen OE. Acetone metabolism in humans during diabetic ketoacidosis. Diabetes. 1986;35:668–74.
Lyles GA, Chalmers J. The metabolism of aminoacetone to methylglyoxal by semicarbazide-sensitive amino oxidase in human umbilical artery. Biochem Pharmacol. 1992;43:1409–14.
Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984;101:527–37.
Thornalley PJ. Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem J. 1988;254:751–5.
Schauenstein E, Esterbauer H, Zollner H. Aldehydes in biological systems. London: Pion Ltd; 1977. p. 112–57.
Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complication. N Engl J Med. 1988;318:1315–21.
Ono Y, Aoki S, Ohnishi K, Yasuda T, Kawano K, Tsukada Y. Increased serum levels of advanced glycation end-products and diabetic complications. Diabetes Res Clin Pract. 1998;41:131–7.
Pierluigi P, Piercarlo M, Colomba F, Valentina M, Enzo E. Association analysis of the functional Ala111Glu polymorphism of glyoxalase I gene. Neurosci Lett. 2006;396(2):163–6.
Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci. 2005;109(2):143–59.
Evans M, Khan N, Rees A. Diabetic dyslipidaemia and coronary heart disease: new perspectives. Curr Opin Lipidol. 1999;10:387–91.
Sahadevan M, Kasiske BL. Hyperlipidemia in kidney disease: causes and consequences. Curr Opin Nephrol Hypertens. 2002;11:323–9.
Acknowledgments
We would like to thank the Regional Delegate of health and the public medical team in Tangier and Tetouan for their services and assistance in blood sampling. We would like to also thank Dr. Ahmed Lazrak (University of Alabama at Birmingham, Department of Anesthesiology) for helpful revision and Dr. Abdeloihid Elmoussaoui (FST of Tangier) for helpful statistical analysis. This study is supported by the research funds of the University of Abdelmalek Essaâdi, Tangier, Morocco.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Meriem Hamoudane and Amina Amakran are contributed equally to this work
Rights and permissions
About this article
Cite this article
Hamoudane, M., Amakran, A., Bakrim, N. et al. Decreased blood levels of glyoxalase I and diabetic complications. Int J Diabetes Dev Ctries 35 (Suppl 3), 496–501 (2015). https://doi.org/10.1007/s13410-014-0237-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-014-0237-4