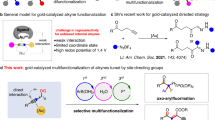
Abstract
The Heck reaction has been considered a robust method for the cross-coupling reaction of olefins and aryl halides to yield alkenes. However, the most significant requirement is the necessity of electronically biased olefins and the requirement of directing group to control the regioselectivity of the Heck reaction. The research group of Patil and Gandon recently documented the gold-catalyzed Heck reaction, demonstrating the utilization of simple aliphatic alkenes as substrates. This approach does not need electronically biased olefins and offers a distinct regioselectivity when compared to the Heck reaction catalyzed by other transition metal catalysts.






Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Heck RF (1982) Palladium‐catalyzed vinylation of organic halides. Organic Reactions 345–390. https://doi.org/10.1002/0471264180.or027.02
Berteina-Raboin S (2019) Catalyzed Mizoroki-Heck reaction or C-H activation. Catalysts 9:925. https://doi.org/10.3390/catal9110925
Heck RF (1979) Palladium-catalyzed reactions of organic halides with olefins. Acc Chem Res 12:146–151. https://doi.org/10.1021/ar50136a006
Crisp GT (1998) Variations on a theme - recent developments on the mechanism of the Heck reaction and their implications for synthesis. Chem Soc Rev 27:427–436. https://doi.org/10.1039/a827427z
Knowles JP, Whiting A (2007) The Heck-Mizoroki cross-coupling reaction: a mechanistic perspective. Org Biomol Chem 5:31–44. https://doi.org/10.1039/b611547k
Werner EW, Mei T-S, Burckle AJ, Sigman MS (2012) Enantioselective Heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science 338:1455–1458. https://doi.org/10.1126/science.1229208
Xu L, Hilton MJ, Zhang X, Norrby P-O, Wu Y-D, Sigman MS, Wiest O (2014) Mechanism, reactivity, and selectivity in palladium-catalyzed redox-relay Heck arylations of alkenyl alcohols. J Am Chem Soc 136:1960–1967. https://doi.org/10.1021/ja4109616
Werner EW, Sigman MS (2010) A highly selective and general palladium catalyst for the oxidative Heck reaction of electronically nonbiased olefins. J Am Chem Soc 132:13981–13983. https://doi.org/10.1021/ja1060998
Werner EW, Sigman MS (2011) Operationally simple and highly (E)-styrenyl-selective Heck reactions of electronically nonbiased olefins. J Am Chem Soc 133:9692–9695. https://doi.org/10.1021/ja203164p
Delcamp JH, Brucks AP, White MC (2008) A general and highly selective chelate-controlled intermolecular oxidative Heck reaction. J Am Chem Soc 130:11270–11271. https://doi.org/10.1021/ja804120r
Romine AM, Yang KS, Karunananda MK, Chen JS, Engle KM (2019) Synthetic and mechanistic studies of a versatile heteroaryl thioether directing group for Pd(II) catalysis. ACS Cat 9:7626–7640. https://doi.org/10.1021/acscatal.9b01471
Yang S, Liu L, Zhou Z, Huang Z, Zhao Y (2020) Palladium-catalyzed direct C-H arylation of 3-butenoic acid derivatives. Org Lett 23:296–299. https://doi.org/10.1021/acs.orglett.0c03773
Font P, Ribas X (2021) Fundamental basis for implementing oxidant-free Au(I)/Au(III) catalysis. Eur J of Inorg Chem 2021:2556–2569. https://doi.org/10.1002/ejic.202100301
Bhoyare VW, Tathe AG, Das A, Chintawar CC, Patil NT (2021) The interplay of carbophilic activation and Au(i)/Au(iii) catalysis: an emerging technique for 1,2-difunctionalization of C-C multiple bonds. Che Soc Rev 50:10422–10450. https://doi.org/10.1039/d0cs00700e
Hopkinson MN, Gee AD, Gouverneur V (2011) AuI/AuIII catalysis: an alternative approach for C-C oxidative coupling. Chem Eur J 17:8248–8262. https://doi.org/10.1002/chem.201100736
Zeineddine A, Estévez L, Mallet-Ladeira S, Miqueu K, Amgoune A, Bourissou D (2017) Rational development of catalytic Au(I)/Au(III) arylation involving mild oxidative addition of aryl halides. Nat Commun 8:565. https://doi.org/10.1038/s41467-017-00672-8
Rodriguez J, Zeineddine A, Sosa Carrizo ED, Miqueu K, Saffon-Merceron N, Amgoune A, Bourissou D (2019) Catalytic Au(i)/Au(iii) arylation with the hemilabile MeDalphos ligand: unusual selectivity for electron-rich iodoarenes and efficient application to indoles. Chem Sci 10:7183–7192. https://doi.org/10.1039/c9sc01954e
Akram MO, Das A, Chakrabarty I, Patil NT (2019) Ligand-enabled gold-catalyzed C(sp2)–N cross-coupling reactions of aryl iodides with amines. Org Lett 21:8101–8105. https://doi.org/10.1021/acs.orglett.9b03082
Rodriguez J, Adet N, Saffon-Merceron N, Bourissou D (2020) Au(i)/Au(iii)-catalyzed C-N coupling. Chem Commun 56:94–97. https://doi.org/10.1039/c9cc07666b
Mudshinge SR, Yang Y, Xu B, Hammond GB, Lu Z (2022) Gold (I/III)-Catalyzed trifluoromethylthiolation and trifluoromethylselenolation of organohalides. Angew Chem Intl Ed 61:e202115687. https://doi.org/10.1002/anie.202115687
Tathe AG, Patil NT (2022) Ligand-enabled gold-catalyzed C(sp2)–S cross-coupling reactions. Org Lett 24:4459–4463. https://doi.org/10.1021/acs.orglett.2c01692
Chen G, Xu B (2023) Hydrogen bond donor and unbalanced ion pair promoter-assisted gold-catalyzed carbon–oxygen cross-coupling of (hetero)aryl iodides with alcohols. ACS Cat 13:1823–1829. https://doi.org/10.1021/acscatal.2c05890
Das A, Patil NT (2023) Ligand-enabled gold-catalyzed C(sp2)–O cross-coupling reactions. ACS Cat 13:3847–3853. https://doi.org/10.1021/acscatal.3c00338
Li W, Chen Y, Chen Y, Xia S, Chang W, Zhu C, Houk KN, Liang Y, Xie J (2023) Site-selective arylation of carboxamides from unprotected peptides. J Am Chem Soc 145:14865–14873. https://doi.org/10.1021/jacs.3c03840
Urvashi U, Mishra S, Patil NT (2023) Gold-catalyzed alkenylation and arylation of phosphorothioates. Chem Sci 14:12134–13139. https://doi.org/10.1039/d3sc04888h
Xie J, Li J, Weingand V, Rudolph M, Hashmi ASK (2016) Intermolecular photocatalyzed Heck-like coupling of unactivated alkyl bromides by a dinuclear gold complex. Chem Eur J 22:12646–12650. https://doi.org/10.1002/chem.201602939
Wei C, Zhang L, Xia Z (2023) Hemilabile P, N-ligand-assisted gold-catalyzed Heck reaction of aryl and styryl iodides with styrenes. Org Lett 25:6808–6812. https://doi.org/10.1021/acs.orglett.3c02244
Rigoulet M, Thillaye du Boullay O, Amgoune A, Bourissou D (2020) Gold(I)/Gold(III) Catalysis that merges oxidative addition and π-alkene activation. Angew Chem Int Ed 59:16625–16630. https://doi.org/10.1002/anie.202006074
Chintawar CC, Yadav AK, Patil NT (2020) Gold-catalyzed 1,2-diarylation of alkenes. Angew Chem Int Ed 59:11808–11813. https://doi.org/10.1002/anie.202002141
Zhang S, Wang C, Ye X, Shi X (2020) Intermolecular alkene difunctionalization via gold-catalyzed oxyarylation. Angew Chem Int Ed 59:20470–20474. https://doi.org/10.1002/anie.202009636
Tathe AG, Chintawar CC, Bhoyare VW, Patil NT (2020) Ligand-enabled gold-catalyzed 1,2-heteroarylation of alkenes. Chem Commun 56:9304–9307. https://doi.org/10.1039/d0cc03707a
Tathe AG, Urvashi YAK, Chintawar CC, Patil NT (2021) Gold-catalyzed 1,2-aminoarylation of alkenes with external amines. ACS Cat 11:4576–4582. https://doi.org/10.1021/acscatal.1c00789
Chintawar CC, Bhoyare VW, Mane MV, Patil NT (2022) Enantioselective Au(I)/Au(III) redox catalysis enabled by chiral (P, N)-ligands. J Am Chem Soc 144:7089–7095. https://doi.org/10.1021/jacs.2c02799
Ye X, Wang C, Zhang S, Tang Q, Wojtas L, Li M, Shi X (2022) Chiral hemilabile P, N-ligand-assisted gold redox catalysis for enantioselective alkene aminoarylation. Chem Eur J 28:e202201018. https://doi.org/10.1002/chem.202201018
Sancheti SP, Singh Y, Mane MV, Patil NT (2023) Gold-catalyzed 1,2-dicarbofunctionalization of alkynes with organohalides. Angew Chem Int Ed 62:e202310493. https://doi.org/10.1002/anie.202310493
Kumar A, Das A, Patil NT (2023) Gold-catalyzed aryl-alkenylation of alkenes. Org Lett 25:2934–2938. https://doi.org/10.1021/acs.orglett.3c01044
Joost M, Amgoune A, Bourissou D (2015) Reactivity of gold complexes towards elementary organometallic reactions. Angew Chem Int Ed 54:15022–15045. https://doi.org/10.1002/anie.201506271
Rekhroukh F, Estevez L, Mallet-Ladeira S, Miqueu K, Amgoune A, Bourissou D (2016) β-hydride Elimination at low-coordinate gold(III) centers. J Am Chem Soc 138:11920–11929. https://doi.org/10.1021/jacs.6b07035
Mankad NP, Toste FD (2012) C(sp3)–F reductive elimination from alkylgold(iii) fluoride complexes. Chem Sci 3:72–76. https://doi.org/10.1039/c1sc00515d
Kumar R, Krieger J-P, Gómez-Bengoa E, Fox T, Linden A, Nevado C (2017) The first gold(III) formate: evidence for β-hydride elimination. Angew Chem Int Ed 56:12862–12865. https://doi.org/10.1002/anie.201705557
Roşca D-A, Smith DA, Hughes DL, Bochmann M (2012) A thermally stable gold(III) hydride: synthesis, reactivity, and reductive condensation as a route to gold(II) complexes. Angew Chem Int Ed 51:10643–10646. https://doi.org/10.1002/anie.201206468
Rekhroukh F, Blons C, Estévez L, Mallet-Ladeira S, Miqueu K, Amgoune A, Bourissou D (2017) Gold(iii)–arene complexes by insertion of olefins into gold–aryl bonds. Chem Sci 8:4539–4545. https://doi.org/10.1039/c7sc00145b
Serra J, Font P, Sosa Carrizo ED, Mallet-Ladeira S, Massou S, Parella T, Miqueu K, Amgoune A, Ribas X, Bourissou D (2018) Cyclometalated gold(iii) complexes: noticeable differences between (N, C) and (P, C) ligands in migratory insertion. Chem Sci 9:3932–3940. https://doi.org/10.1039/c7sc04899h
Langseth E, Nova A, Tråseth EA, Rise F, Øien S, Heyn RH, Tilset M (2014) A gold exchange: a mechanistic study of a reversible, formal ethylene insertion into a gold(III)–oxygen bond. J Am Chem Soc 136:10104–10115. https://doi.org/10.1021/ja504554u
Cadge JA, Gates PJ, Bower JF, Russell CA (2022) Migratory insertion of CO into a Au–C bond. J Am Chem Soc 144:19719–19725. https://doi.org/10.1021/jacs.2c10432
Bhoyare VW, Sosa Carrizo ED, Chintawar CC, Gandon V, Patil NT (2023) Gold-catalyzed Heck reaction. J Am Chem Soc 145:8810–8816. https://doi.org/10.1021/jacs.3c02544
Bhoyare VW, Tathe AG, Gandon V, Patil NT (2023) Unlocking the chain-walking process in gold catalysis. Angew Chem Int Ed 62:e202312786. https://doi.org/10.1002/anie.202312786
Budzelaar PHM, Bochmann M, Landrini M, Rocchigiani L (2024) Gold-catalysed Heck reactions: fact or fiction? https://doi.org/10.26434/chemrxiv-2024-3rldf
Acknowledgements
MBT thanks SERB, New Delhi, for providing a postdoctoral fellowship (PDF/2022/002920)
Funding
Generous financial support by the Science and Engineering Research Board (SERB), New Delhi (CRG/2022/000195, SCP/2022/000063, and JCB/2022/000052), is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
M. B. T. wrote the manuscript. N.T.P. proofread and endorsed the content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thoke, M.B., Patil, N.T. Unusual selectivity in gold-catalyzed intermolecular Heck reactions. Gold Bull 56, 159–165 (2023). https://doi.org/10.1007/s13404-024-00342-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-024-00342-w