Abstract
Purpose
Sunitinib is a recommended drug for metastatic renal cell carcinoma (RCC). However, the therapeutic potential of sunitinib is impaired by toxicity and resistance. Therefore, we seek to explore a combinatorial strategy to improve sunitinib efficacy of low-toxicity dose for better clinical application.
Methods
We screen synergistic reagents of sunitinib from a compound library containing 1374 FDA-approved drugs by in vitro cell viability evaluation. The synergistically antiproliferative and proapoptotic effects were demonstrated on in vitro and in vivo models. The molecular mechanism was investigated by phosphoproteomics, co-immunoprecipitation, immunofluorescence and western-blot assays, etc.
Results
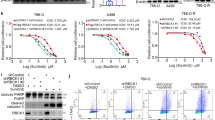
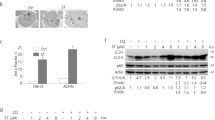
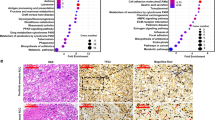
From the four-step screening, nilotinib stood out as a potential synergistic killer combined with sunitinib. Subsequent functional evaluation demonstrated that nilotinib and sunitinib synergistically inhibit RCC cell proliferation and promote apoptosis in vitro and in vivo. Mechanistically, nilotinib activates E3-ligase HUWE1 and in combination with sunitinib renders MCL-1 for degradation via proteasome pathway, resulting in the release of Beclin-1 from MCL-1/Beclin-1 complex. Subsequently, Beclin-1 induces complete autophagy flux to promote antitumor effect.
Conclusion
Our findings revealed that a novel mechanism that nilotinib in combination with sunitinib overcomes sunitinib resistance in RCC. Therefore, this novel rational combination regimen provides a promising therapeutic avenue for metastatic RCC and rationale for evaluating this combination clinically.







Similar content being viewed by others
Data availability
All data are available within the article, supplementary information, or available from the corresponding author upon reasonable request.
Abbreviations
- AXL:
-
AXL receptor tyrosine kinase
- BCL-2:
-
BCL2 apoptosis regulator
- BCL-XL:
-
Apoptosis regulator Bcl-X
- c-Kit:
-
Stem cell growth factor receptor Kit
- FDA:
-
Food and drug administration
- FLT-3:
-
Fms related receptor tyrosine kinase 3
- HBSS:
-
Hanks’ balance salt solution
- HUWE1:
-
HECT, UBA and WWE domain containing E3 ubiquitin protein ligase 1
- IP:
-
Immunoprecipitation
- IF:
-
Immunofluorescence
- LC3:
-
Microtubule associated protein 1 light chain 3 alpha
- MCL-1:
-
Myeloid cell leukemia 1
- MET:
-
Tyrosine-protein kinase Met
- mTORC1:
-
Mammalian target of rapamycin complex 1
- PDGFR:
-
Platelet-derived growth factor receptor
- PFS:
-
Progression free survival
- RCC:
-
Renal cell carcinoma
- TKI:
-
Tyrosine kinase inhibitor
- USP9X:
-
Ubiquitin specific peptidase 9 X-linked
- VEGFR:
-
Vascular endothelial growth factor receptor
- WB:
-
Western blot
References
B. Shuch, A. Amin, A.J. Armstrong, J.N. Eble, V. Ficarra, A. Lopez-Beltran et al., Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Europ. Urol. 67, 85–97 (2015). https://doi.org/10.1016/j.eururo.2014.04.029
J.S. Lam, J.T. Leppert, A.S. Belldegrun, R.A. Figlin, Novel approaches in the therapy of metastatic renal cell carcinoma. World J. Urol. 23, 202–212 (2005). https://doi.org/10.1007/s00345-004-0466-0
N.K. Janzen, H.L. Kim, R.A. Figlin, A.S. Belldegrun, Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urologic Clini. North Am. 30, 843–852 (2003). https://doi.org/10.1016/s0094-0143(03)00056-9
A.M. Molina, X. Lin, B. Korytowsky, E. Matczak, M.J. Lechuga, R. Wiltshire et al., Sunitinib objective response in metastatic renal cell carcinoma: analysis of 1059 patients treated on clinical trials. Eur. J. Cancer 50, 351–358 (2014). https://doi.org/10.1016/j.ejca.2013.08.021
S. Bracarda, R. Iacovelli, L. Boni, M. Rizzo, L. Derosa, M. Rossi et al., Sunitinib administered on 2/1 schedule in patients with metastatic renal cell carcinoma: the RAINBOW analysis. Ann. Oncol. 26, 2107–2113 (2015). https://doi.org/10.1093/annonc/mdv315
M.E. Gore, C. Szczylik, C. Porta, S. Bracarda, G.A. Bjarnason, S. Oudard et al., Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 10, 757–763 (2009). https://doi.org/10.1016/s1470-2045(09)70162-7
B.E. Houk, C.L. Bello, B. Poland, L.S. Rosen, G.D. Demetri, R.J. Motzer, Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother. Pharmacol. 66, 357–371 (2010). https://doi.org/10.1007/s00280-009-1170-y
D.R. Feldman, M.S. Baum, M.S. Ginsberg, H. Hassoun, C.D. Flombaum, S. Velasco et al., Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 27, 1432–1439 (2009). https://doi.org/10.1200/jco.2008.19.0108
P.H. Patel, P.L. Senico, R.E. Curiel, R.J. Motzer, Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. Clin. Genitourinary Cancer 7, 24–27 (2009). https://doi.org/10.3816/CGC.2009.n.004
A. Amin, E.R. Plimack, M.S. Ernstoff, L.D. Lewis, T.M. Bauer, D.F. McDermott et al., Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study. J. Immunother. Cancer 6 (2018). https://doi.org/10.1186/s40425-018-0420-0
M. Elgendy, A.K. Abdel-Aziz, S.L. Renne, V. Bornaghi, G. Procopio, M. Colecchia et al., Dual modulation of MCL-1 and mTOR determines the response to sunitinib. J. Clin. Investig. 127, 153–168 (2017). https://doi.org/10.1172/jci84386
S. Giuliano, Y. Cormerais, M. Dufies, R. Grepin, P. Colosetti, A. Belaid et al., Resistance to sunitinib in renal clear cell carcinoma results from sequestration in lysosomes and inhibition of the autophagic flux. Autophagy 11, 1891–1904 (2015). https://doi.org/10.1080/15548627.2015.1085742
L. DeVorkin, M. Hattersley, P. Kim, J. Ries, J. Spowart, M.S. Anglesio et al., Autophagy inhibition enhances sunitinib efficacy in clear cell ovarian carcinoma. Mol. Cancer Res. 15, 250–258 (2017). https://doi.org/10.1158/1541-7786.Mcr-16-0132
T. Ikeda, K. Ishii, Y. Saito, M. Miura, A. Otagiri, Y. Kawakami et al., Inhibition of autophagy enhances sunitinib-induced cytotoxicity in rat pheochromocytoma PC12 cells. J. Pharmacol. Sci. 121, 67–73 (2013). https://doi.org/10.1254/jphs.12158FP
T. Wiedmer, A. Blank, S. Pantasis, L. Normand, R. Bill, P. Krebs et al., Autophagy inhibition improves sunitinib efficacy in pancreatic neuroendocrine tumors via a lysosome-dependent mechanism. Mol. Cancer Ther. 16, 2502–2515 (2017). https://doi.org/10.1158/1535-7163.Mct-17-0136
K.J. Gotink, H.J. Broxterman, M. Labots, R.R. de Haas, H. Dekker, R.J. Honeywell et al., Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clin. Cancer Res. 17, 7337–7346 (2011). https://doi.org/10.1158/1078-0432.ccr-11-1667
M. Elgendy, C. Sheridan, G. Brumatti, S.J. Martin, Oncogenic ras-induced expression of noxa and beclin-1 promotes autophagic cell death and limits clonogenic survival. Molecular Cell 42, 23–35 (2011). https://doi.org/10.1016/j.molcel.2011.02.009
M.C. Maiuri, G. Le Toumelin, A. Criollo, J.C. Rain, F. Gautier, P. Juin et al., Functional and physical interaction between Bcl-X-L and a BH3-like domain in Beclin-1. EMBO J. 26, 2527–2539 (2007). https://doi.org/10.1038/sj.emboj.7601689
M. Elgendy, M. Ciro, A.K. Abdel-Aziz, G. Belmonte, R. Dal Zuffo, C. Mercurio et al., Beclin 1 restrains tumorigenesis through Mcl-1 destabilization in an autophagy-independent reciprocal manner. Nat. Commun. 5 (2014). https://doi.org/10.1038/ncomms6637
J.R. Wisniewski, A. Zougman, N. Nagaraj, M. Mann, Universal sample preparation method for proteome analysis. Nature Methods 6, 359–U360 (2009). https://doi.org/10.1038/nmeth.1322
M. Zarei, A. Sprenger, M. Rackiewicz, J. Dengjel, Fast and easy phosphopeptide fractionation by combinatorial ERLIC-SCX solid-phase extraction for in-depth phosphoproteome analysis. Nature Protocols 11, 37–45 (2016). https://doi.org/10.1038/nprot.2015.134
T.C. Chou, P. Talalay, Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22, 27–55 (1984). https://doi.org/10.1016/0065-2571(84)90007-4
Z.J. Jin, About the evaluation of drug combination. Acta Pharmacol. Sin. 25, 146–147 (2004)
D. Huang, Y. Ding, Y. Li, W.M. Luo, Z.F. Zhang, J. Snider et al., Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res. 70, 1053–1062 (2010). https://doi.org/10.1158/0008-5472.Can-09-3722
Q. Ding, X. He, J.M. Hsu, W. Xia, C.T. Chen, L.Y. Li et al., Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol. Cell Biol. 27, 4006–4017 (2007). https://doi.org/10.1128/mcb.00620-06
A.M. Domina, J.A. Vrana, M.A. Gregory, S.R. Hann, R.W. Craig, MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene 23, 5301–5315 (2004). https://doi.org/10.1038/sj.onc.1207692
W.T. Tai, C.W. Shiau, H.L. Chen, C.Y. Liu, C.S. Lin, A.L. Cheng et al., Mcl-1-dependent activation of Beclin 1 mediates autophagic cell death induced by sorafenib and SC-59 in hepatocellular carcinoma cells. Cell Death Dis. 4, e485 (2013). https://doi.org/10.1038/cddis.2013.18
G. Marino, M. Niso-Santano, E.H. Baehrecke, G. Kroemer, Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 15, 81–94 (2014). https://doi.org/10.1038/nrm3735
S. Giuliano, Y. Cormerais, M. Dufies, R. Grépin, P. Colosetti, A. Belaid et al., Resistance to sunitinib in renal clear cell carcinoma results from sequestration in lysosomes and inhibition of the autophagic flux. Autophagy 11, 1891–1904 (2015). https://doi.org/10.1080/15548627.2015.1085742
D.J. Klionsky, A.K. Abdel-Aziz, S. Abdelfatah, M. Abdellatif, A. Abdoli, S. Abel et al., Guidelines for the use and interpretation of assays for monitoring autophagy (4th edn). Autophagy 17, 1–382 (2021). https://doi.org/10.1080/15548627.2020.1797280
R.J. Motzer, E. Jonasch, N. Agarwal, A. Alva, M. Baine, K. Beckermann et al., Kidney cancer, version 3.2022. J. Natl. Compr. Cancer Network 20, 71–89 (2022). https://doi.org/10.6004/jnccn.2022.0001
L. Qu, J. Ding, C. Chen, Z.J. Wu, B. Liu, Y. Gao et al., Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell 29, 653–668 (2016). https://doi.org/10.1016/j.ccell.2016.03.004
F. Shojaei, J.H. Lee, B.H. Simmons, A. Wong, C.O. Esparza, P.A. Plumlee et al., HGF/c-met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 70, 10090–10100 (2010). https://doi.org/10.1158/0008-5472.Can-10-0489
L. Zhou, X.D. Liu, M. Sun, X. Zhang, P. German, S. Bai et al., Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 35, 2687–2697 (2016). https://doi.org/10.1038/onc.2015.343
T.K. Choueiri, S. Halabi, B.L. Sanford, O. Hahn, M.D. Michaelson, M.K. Walsh et al., Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J. Clin. Oncol. 35, 591 (2017). https://doi.org/10.1200/jco.2016.70.7398
J. Deng, Q. Zhang, L.P. Lv, P. Ma, Y.Y. Zhang, N. Zhao et al., Identification of an autophagy-related gene signature for predicting prognosis and immune activity in pancreatic adenocarcinoma. Sci. Rep. 12 (2022). https://doi.org/10.1038/s41598-022-11050-w
Y. Ekim, S. Kara, B. Gencer, T. Karaca, Efficacy of sunitinib, sunitinib-hesperetin, and sunitinib-doxycycline combinations on experimentally-induced corneal neovascularization. Curr. Eye Res. 44, 590–598 (2019). https://doi.org/10.1080/02713683.2019.1584320
F. Rossari, F. Minutolo, E. Orciuolo, Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. J. Hematol. Oncol. 11 (2018). https://doi.org/10.1186/s13045-018-0624-2
D.B. Mendel, A.D. Laird, X.H. Xin, S.G. Louie, J.G. Christensen, G.M. Li et al., In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 9, 327–337 (2003)
S. Takasaki, Y. Kawasaki, M. Kikuchi, M. Tanaka, M. Suzuka, A. Noda et al., Relationships between sunitinib plasma concentration and clinical outcomes in Japanese patients with metastatic renal cell carcinoma. Int. J. Clin. Oncol. 23, 936–943 (2018). https://doi.org/10.1007/s10147-018-1302-7
M. Golemovic, S. Verstovsek, F. Giles, J. Cortes, T. Manshouri, P.W. Manley et al., AMN107, a novel aminopyrimidine inhibitor of Bcr-Abl, has in vitro activity against imatinib-resistant chronic myeloid leukemia. Clin. Cancer Res. 11, 4941–4947 (2005). https://doi.org/10.1158/1078-0432.Ccr-04-2601
F. Strappazzon, A. Di Rita, A. Peschiaroli, P.P. Leoncini, F. Locatelli, G. Melino et al., HUWE1 controls MCL1 stability to unleash AMBRA1-induced mitophagy. Cell Death Differ. 27, 1155–1168 (2020). https://doi.org/10.1038/s41418-019-0404-8
Acknowledgements
Not applicable.
Funding
This project was supported by the National Natural Science Foundation of China (NSFC) (No. 82172812 to R.L. and No. 82272743 to X.Y.), The Natural Science Foundation of Guangdong Province (No. 2022A1515012321 to H.H. and No. 2021A1515012496 to X.Y.), The Enterprise United Foundation of Guangdong Province (No. 2021A1515220182 to H.H.).
Author information
Authors and Affiliations
Contributions
Study concept and design: X.Y., R.L. and H.H.; Performed in vitro functional studies and analyzed data: T.L., X.C. and X.Y.; Performed animal experiments and analyzed data: T.L., R.Y. and C.W.; Generated critical cellular tools: T.L. and X.C.; Performed mechanistical studies and analyzed data: T.L., X.Y., X.C. and Y.L.; Statistic analysis: T.L. and X.Y.; Supervision of data: X.B.; Analysis and interpretation of data and writing of the manuscript: T.L., X.Y., R.L, and H.H. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Animal experiments were approved by the Sun Yat-Sen University Cancer Center Institutional Animal Care and Usage Committee following the Animal Welfare and Rights in China (Approval number: L102012019040I). All the experiments were conducted in the Laboratory Animal Center of Sun Yat-sen University.
Consent for publication
Not applicable.
Conflict of interest
None of the authors have any potential conflicts to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, T., Yue, X., Chen, X. et al. Nilotinib in combination with sunitinib renders MCL-1 for degradation and activates autophagy that overcomes sunitinib resistance in renal cell carcinoma. Cell Oncol. (2024). https://doi.org/10.1007/s13402-024-00927-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s13402-024-00927-9