Abstract
Background
Ferroptosis, a newly form of regulated cell death (RCD), is characterized by iron dyshomeostasis and unrestricted lipid peroxidation. Emerging evidence depicts a pivotal role for ferroptosis in driving some pathological processes, especially in cancer. Triggering ferroptosis can suppress tumor growth and induce an anti-tumor immune response, denoting the therapeutic promises for targeting ferroptosis in the management of cancer. As an autophagic phenomenon, ferritinophagy is critical to induce ferroptosis by degradation of ferritin to release intracellular free iron. Recently, a great deal of effort has gone into designing and developing anti-cancer strategies based on targeting ferritinophagy to induce ferroptosis.
Conclusion
This review delineates the regulatory mechanism of ferritinophagy firstly and summarizes the role of ferritinophagy-induced ferroptosis in cancer. Moreover, the strategies targeting ferritinophagy to induce ferroptosis are highlighted to unveil the therapeutic value of ferritinophagy as a target to manage cancer. Finally, the future research directions on how to cope with the challenges in developing ferritinophagy promoters into clinical therapeutics are discussed.
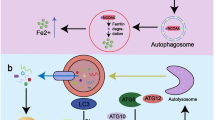
Graphical abstract






Similar content being viewed by others
Data availability
Not applicable.
References
M. Gao, X. Jiang, To eat or not to eat-the metabolic flavor of ferroptosis. Curr. Opin. Cell Biol. 51, 58–64 (2018)
L. Galluzzi, I. Vitale, S.A. Aaronson, J.M. Abrams, D. Adam, P. Agostinis, E.S. Alnemri, L. Altucci, I. Amelio, D.W. Andrews, M. Annicchiarico-Petruzzelli, A.V. Antonov, E. Arama, E.H. Baehrecke, N.A. Barlev, N.G. Bazan, F. Bernassola, M.J.M. Bertrand, K. Bianchi, M.V. Blagosklonny, K. Blomgren, C. Borner, P. Boya, C. Brenner, M. Campanella, E. Candi, D. Carmona-Gutierrez, F. Cecconi, F.K. Chan, N.S. Chandel, E.H. Cheng, J.E. Chipuk, J.A. Cidlowski, A. Ciechanover, G.M. Cohen, M. Conrad, J.R. Cubillos-Ruiz, P.E. Czabotar, V. D’Angiolella, T.M. Dawson, V.L. Dawson, V. De Laurenzi, R. De Maria, K.M. Debatin, R.J. DeBerardinis, M. Deshmukh, N. Di Daniele, F. Di Virgilio, V.M. Dixit, S.J. Dixon, C.S. Duckett, B.D. Dynlacht, W.S. El-Deiry, J.W. Elrod, G.M. Fimia, S. Fulda, A.J. García-Sáez, A.D. Garg, C. Garrido, E. Gavathiotis, P. Golstein, E. Gottlieb, D.R. Green, L.A. Greene, H. Gronemeyer, A. Gross, G. Hajnoczky, J.M. Hardwick, I.S. Harris, M.O. Hengartner, C. Hetz, H. Ichijo, M. Jäättelä, B. Joseph, P.J. Jost, P.P. Juin, W.J. Kaiser, M. Karin, T. Kaufmann, O. Kepp, A. Kimchi, R.N. Kitsis, D.J. Klionsky, R.A. Knight, S. Kumar, S.W. Lee, J.J. Lemasters, B. Levine, A. Linkermann, S.A. Lipton, R.A. Lockshin, C. López-Otín, S.W. Lowe, T. Luedde, E. Lugli, M. MacFarlane, F. Madeo, M. Malewicz, W. Malorni, G. Manic, J.C. Marine, S.J. Martin, J.C. Martinou, J.P. Medema, P. Mehlen, P. Meier, S. Melino, E.A. Miao, J.D. Molkentin, U.M. Moll, C. Muñoz-Pinedo, S. Nagata, G. Nuñez, A. Oberst, M. Oren, M. Overholtzer, M. Pagano, T. Panaretakis, M. Pasparakis, J.M. Penninger, D.M. Pereira, S. Pervaiz, M.E. Peter, M. Piacentini, P. Pinton, J.H.M. Prehn, H. Puthalakath, G.A. Rabinovich, M. Rehm, R. Rizzuto, C.M.P. Rodrigues, D.C. Rubinsztein, T. Rudel, K.M. Ryan, E. Sayan, L. Scorrano, F. Shao, Y. Shi, J. Silke, H.U. Simon, A. Sistigu, B.R. Stockwell, A. Strasser, G. Szabadkai, S.W.G. Tait, D. Tang, N. Tavernarakis, A. Thorburn, Y. Tsujimoto, B. Turk, T. VandenBerghe, P. Vandenabeele, M.G. Vander Heiden, A. Villunger, H.W. Virgin, K.H. Vousden, D. Vucic, E.F. Wagner, H. Walczak, D. Wallach, Y. Wang, J.A. Wells, W. Wood, J. Yuan, Z. Zakeri, B. Zhivotovsky, L. Zitvogel, G. Melino, G. Kroemer, Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018)
E. Koren, Y. Fuchs, Modes of regulated cell death in cancer. Cancer Discov. 11, 245–265 (2021)
S.J. Dixon, B.R. Stockwell, The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10, 9–17 (2014)
D. Tang, G. Kroemer, Ferroptosis. Curr. Biol. 30, R1292-r1297 (2020)
Y. Xie, W. Hou, X. Song, Y. Yu, J. Huang, X. Sun, R. Kang, D. Tang, Ferroptosis: process and function. Cell Death Differ. 23, 369–379 (2016)
S.J. Dixon, K.M. Lemberg, M.R. Lamprecht, R. Skouta, E.M. Zaitsev, C.E. Gleason, D.N. Patel, A.J. Bauer, A.M. Cantley, W.S. Yang, B. Morrison 3rd., B.R. Stockwell, Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012)
W. Wang, M. Green, J.E. Choi, M. Gijón, P.D. Kennedy, J.K. Johnson, P. Liao, X. Lang, I. Kryczek, A. Sell, H. Xia, J. Zhou, G. Li, J. Li, W. Li, S. Wei, L. Vatan, H. Zhang, W. Szeliga, W. Gu, R. Liu, T.S. Lawrence, C. Lamb, Y. Tanno, M. Cieslik, E. Stone, G. Georgiou, T.A. Chan, A. Chinnaiyan, W. Zou, CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274 (2019)
J.P. FriedmannAngeli, D.V. Krysko, M. Conrad, Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 19, 405–414 (2019)
H. Chang, Z. Zou, Targeting autophagy to overcome drug resistance: further developments. J. Hematol. Oncol. 13, 159 (2020)
B. Levine, N. Mizushima, H.W. Virgin, Autophagy in immunity and inflammation. Nature 469, 323–335 (2011)
M. Gao, P. Monian, Q. Pan, W. Zhang, J. Xiang, X. Jiang, Ferroptosis is an autophagic cell death process. Cell Res. 26, 1021–1032 (2016)
A. Ajoolabady, H. Aslkhodapasandhokmabad, P. Libby, J. Tuomilehto, G.Y.H. Lip, J.M. Penninger, D.R. Richardson, D. Tang, H. Zhou, S. Wang, D.J. Klionsky, G. Kroemer, J. Ren, Ferritinophagy and ferroptosis in the management of metabolic diseases. Trends Endocrinol. Metab. 32, 444–462 (2021)
G.O. Latunde-Dada, Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 1861, 1893–1900 (2017)
B.R. Stockwell, X. Jiang, W. Gu, Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 30, 478–490 (2020)
D. Tang, X. Chen, R. Kang, G. Kroemer, Ferroptosis: molecular mechanisms and health implications. Cell Res. 31, 107–125 (2021)
Y. Bai, L. Meng, L. Han, Y. Jia, Y. Zhao, H. Gao, R. Kang, X. Wang, D. Tang, E. Dai, Lipid storage and lipophagy regulates ferroptosis. Biochem. Biophys. Res. Commun. 508, 997–1003 (2019)
M. Yang, P. Chen, J. Liu, S. Zhu, G. Kroemer, D.J. Klionsky, M.T. Lotze, H.J. Zeh, R. Kang, D. Tang, Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci. Adv. 5, eaaw2238 (2019)
Z. Wu, Y. Geng, X. Lu, Y. Shi, G. Wu, M. Zhang, B. Shan, H. Pan, J. Yuan, Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 116, 2996–3005 (2019)
M. Gao, J. Yi, J. Zhu, A.M. Minikes, P. Monian, C.B. Thompson, X. Jiang, Role of mitochondria in ferroptosis. Mol. Cell. 73, 354-363.e353 (2019)
M.U. Muckenthaler, S. Rivella, M.W. Hentze, B. Galy, A red carpet for iron metabolism. Cell 168, 344–361 (2017)
K. Gkouvatsos, G. Papanikolaou, K. Pantopoulos, Regulation of iron transport and the role of transferrin. Biochim. Biophys. Acta 1820, 188–202 (2012)
Y. Cheng, O. Zak, P. Aisen, S.C. Harrison, T. Walz, Structure of the human transferrin receptor-transferrin complex. Cell 116, 565–576 (2004)
M.W. Hentze, M.U. Muckenthaler, B. Galy, C. Camaschella, Two to tango: regulation of Mammalian iron metabolism. Cell 142, 24–38 (2010)
S. Puig, L. Ramos-Alonso, A.M. Romero, M.T. Martínez-Pastor, The elemental role of iron in DNA synthesis and repair. Metallomics 9, 1483–1500 (2017)
D.M. Ward, S.M. Cloonan, Mitochondrial Iron in Human Health and Disease. Annu Rev Physiol. 81, 453–482 (2019)
P.M. Harrison, P. Arosio, The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1275, 161–203 (1996)
S. Levi, B. Corsi, M. Bosisio, R. Invernizzi, A. Volz, D. Sanford, P. Arosio, J. Drysdale, A human mitochondrial ferritin encoded by an intronless gene. J. Biol. Chem. 276, 24437–24440 (2001)
B. Corsi, A. Cozzi, P. Arosio, J. Drysdale, P. Santambrogio, A. Campanella, G. Biasiotto, A. Albertini, S. Levi, Human mitochondrial ferritin expressed in HeLa cells incorporates iron and affects cellular iron metabolism. J. Biol. Chem. 277, 22430–22437 (2002)
P. Arosio, L. Elia, M. Poli, Ferritin, cellular iron storage and regulation. IUBMB Life 69, 414–422 (2017)
T.A. Rouault, N. Maio, Biogenesis and functions of mammalian iron-sulfur proteins in the regulation of iron homeostasis and pivotal metabolic pathways. J. Biol. Chem. 292, 12744–12753 (2017)
C. Monaco, R. Visconti, M.V. Barone, G.M. Pierantoni, M.T. Berlingieri, C. De Lorenzo, A. Mineo, G. Vecchio, A. Fusco, M. Santoro, The RFG oligomerization domain mediates kinase activation and re-localization of the RET/PTC3 oncoprotein to the plasma membrane. Oncogene 20, 599–608 (2001)
T. Asano, M. Komatsu, Y. Yamaguchi-Iwai, F. Ishikawa, N. Mizushima, K. Iwai, Distinct mechanisms of ferritin delivery to lysosomes in iron-depleted and iron-replete cells. Mol. Cell Biol. 31, 2040–2052 (2011)
R. Bellelli, M.D. Castellone, T. Guida, R. Limongello, N.A. Dathan, F. Merolla, A.M. Cirafici, A. Affuso, H. Masai, V. Costanzo, D. Grieco, A. Fusco, M. Santoro, F. Carlomagno, NCOA4 transcriptional coactivator inhibits activation of DNA replication origins. Mol. Cell. 55, 123–137 (2014)
T. Gao, K. Brantley, E. Bolu, M.J. McPhaul, RFG (ARA70, ELE1) interacts with the human androgen receptor in a ligand-dependent fashion, but functions only weakly as a coactivator in cotransfection assays. Mol. Endocrinol. 13, 1645–1656 (1999)
W.E. Dowdle, B. Nyfeler, J. Nagel, R.A. Elling, S. Liu, E. Triantafellow, S. Menon, Z. Wang, A. Honda, G. Pardee, J. Cantwell, C. Luu, I. Cornella-Taracido, E. Harrington, P. Fekkes, H. Lei, Q. Fang, M.E. Digan, D. Burdick, A.F. Powers, S.B. Helliwell, S. D’Aquin, J. Bastien, H. Wang, D. Wiederschain, J. Kuerth, P. Bergman, D. Schwalb, J. Thomas, S. Ugwonali, F. Harbinski, J. Tallarico, C.J. Wilson, V.E. Myer, J.A. Porter, D.E. Bussiere, P.M. Finan, M.A. Labow, X. Mao, L.G. Hamann, B.D. Manning, R.A. Valdez, T. Nicholson, M. Schirle, M.S. Knapp, E.P. Keaney, L.O. Murphy, Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 16, 1069–1079 (2014)
J.D. Mancias, X. Wang, S.P. Gygi, J.W. Harper, A.C. Kimmelman, Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509, 105–109 (2014)
M. Gryzik, A. Srivastava, G. Longhi, M. Bertuzzi, A. Gianoncelli, F. Carmona, M. Poli, P. Arosio, Expression and characterization of the ferritin binding domain of Nuclear Receptor Coactivator-4 (NCOA4). Biochim. Biophys. Acta Gen. Subj. 1861, 2710–2716 (2017)
J.D. Mancias, L. Pontano Vaites, S. Nissim, D.E. Biancur, A.J. Kim, X. Wang, Y. Liu, W. Goessling, A.C. Kimmelman, J.W. Harper, Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife 4, e10308 (2015)
K. Li, B. Chen, A. Xu, J. Shen, K. Li, K. Hao, R. Hao, W. Yang, W. Jiang, Y. Zheng, F. Ge, Z. Wang, TRIM7 modulates NCOA4-mediated ferritinophagy and ferroptosis in glioblastoma cells. Redox Biol. 56, 102451 (2022)
D.J. Klionsky, J.M. Cregg, W.A. Dunn Jr., S.D. Emr, Y. Sakai, I.V. Sandoval, A. Sibirny, S. Subramani, M. Thumm, M. Veenhuis, Y. Ohsumi, A unified nomenclature for yeast autophagy-related genes. Dev. Cell 5, 539–545 (2003)
H. Nakatogawa, K. Suzuki, Y. Kamada, Y. Ohsumi, Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell Biol. 10, 458–467 (2009)
Y. Xie, R. Kang, X. Sun, M. Zhong, J. Huang, D.J. Klionsky, D. Tang, Posttranslational modification of autophagy-related proteins in macroautophagy. Autophagy 11, 28–45 (2015)
R. Bellelli, G. Federico, A. Matte’, D. Colecchia, A. Iolascon, M. Chiariello, M. Santoro, L. De Franceschi, F. Carlomagno, NCOA4 deficiency impairs systemic iron homeostasis. Cell Rep. 14, 411–421 (2016)
W. Hou, Y. Xie, X. Song, X. Sun, M.T. Lotze, H.J. Zeh 3rd., R. Kang, D. Tang, Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12, 1425–1428 (2016)
X. Qin, J. Zhang, B. Wang, G. Xu, X. Yang, Z. Zou, C. Yu, Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy 17, 4266–4285 (2021)
C. Kishi-Itakura, I. Koyama-Honda, E. Itakura, N. Mizushima, Ultrastructural analysis of autophagosome organization using mammalian autophagy-deficient cells. J. Cell Sci. 127, 4089–4102 (2014)
J.M. Goodwin, W.E. Dowdle, R. DeJesus, Z. Wang, P. Bergman, M. Kobylarz, A. Lindeman, R.J. Xavier, G. McAllister, B. Nyfeler, G. Hoffman, L.O. Murphy, Autophagy-independent lysosomal targeting regulated by ULK1/2-FIP200 and ATG9. Cell Rep. 20, 2341–2356 (2017)
Z. Zhang, M. Guo, Y. Li, M. Shen, D. Kong, J. Shao, H. Ding, S. Tan, A. Chen, F. Zhang, S. Zheng, RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy 16, 1482–1505 (2020)
V. Lahiri, W.D. Hawkins, D.J. Klionsky, Watch What You (Self-) Eat: Autophagic Mechanisms that Modulate Metabolism. Cell Metab. 29, 803–826 (2019)
D.H. Manz, N.L. Blanchette, B.T. Paul, F.M. Torti, S.V. Torti, Iron and cancer: recent insights. Ann. N. Y. Acad. Sci. 1368, 149–161 (2016)
D. Basuli, L. Tesfay, Z. Deng, B. Paul, Y. Yamamoto, G. Ning, W. Xian, F. McKeon, M. Lynch, C.P. Crum, P. Hegde, M. Brewer, X. Wang, L.D. Miller, N. Dyment, F.M. Torti, S.V. Torti, Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene 36, 4089–4099 (2017)
F. Zhang, W. Wang, Y. Tsuji, S.V. Torti, F.M. Torti, Post-transcriptional modulation of iron homeostasis during p53-dependent growth arrest. J. Biol. Chem. 283, 33911–33918 (2008)
C. Mertens, J. Mora, B. Ören, S. Grein, S. Winslow, K. Scholich, A. Weigert, P. Malmström, C. Forsare, M. Fernö, T. Schmid, B. Brüne, M. Jung, Macrophage-derived lipocalin-2 transports iron in the tumor microenvironment. Oncoimmunology 7, e1408751 (2018)
S. Recalcati, M. Locati, A. Marini, P. Santambrogio, F. Zaninotto, M. De Pizzol, L. Zammataro, D. Girelli, G. Cairo, Differential regulation of iron homeostasis during human macrophage polarized activation. Eur. J. Immunol. 40, 824–835 (2010)
M.A. Bevilacqua, M.C. Faniello, T. Russo, F. Cimino, F. Costanzo, P/CAF/p300 complex binds the promoter for the heavy subunit of ferritin and contributes to its tissue-specific expression. Biochem. J. 335(Pt 3), 521–525 (1998)
Y. Tsuji, N. Akebi, T.K. Lam, Y. Nakabeppu, S.V. Torti, F.M. Torti, FER-1, an enhancer of the ferritin H gene and a target of E1A-mediated transcriptional repression. Mol. Cell Biol. 15, 5152–5164 (1995)
K.J. Wu, A. Polack, R. Dalla-Favera, Coordinated regulation of iron-controlling genes, H-ferritin and IRP2, by c-MYC. Science 283, 676–679 (1999)
O. Kakhlon, Y. Gruenbaum, Z.I. Cabantchik, Repression of ferritin expression modulates cell responsiveness to H-ras-induced growth. Biochem. Soc. Trans. 30, 777–780 (2002)
A. Cozzi, B. Corsi, S. Levi, P. Santambrogio, A. Albertini, P. Arosio, Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: in vivo role of ferritin ferroxidase activity. J. Biol. Chem. 275, 25122–25129 (2000)
S. Rockfield, I. Flores, M. Nanjundan, Expression and function of nuclear receptor coactivator 4 isoforms in transformed endometriotic and malignant ovarian cells. Oncotarget 9, 5344–5367 (2018)
P.A. Shaw, P.V. Rittenberg, T.J. Brown, Activation of androgen receptor-associated protein 70 (ARA70) mRNA expression in ovarian cancer. Gynecol. Oncol. 80, 132–138 (2001)
Y. Peng, C.X. Li, F. Chen, Z. Wang, M. Ligr, J. Melamed, J. Wei, W. Gerald, M. Pagano, M.J. Garabedian, P. Lee, Stimulation of prostate cancer cellular proliferation and invasion by the androgen receptor co-activator ARA70. Am. J. Pathol. 172, 225–235 (2008)
X. Wu, F. Chen, A. Sahin, C. Albarracin, Z. Pei, X. Zou, B. Singh, R. Xu, G. Daniels, Y. Li, J. Wei, M. Blake, R.J. Schneider, P. Cowin, P. Lee, Distinct function of androgen receptor coactivator ARA70α and ARA70β in mammary gland development, and in breast cancer. Breast Cancer Res. Treat. 128, 391–400 (2011)
P.S. Steeg, Targeting metastasis. Nat. Rev. Cancer 16, 201–218 (2016)
A. Hoshino, D. Lyden, Metastasis: lymphatic detours for cancer. Nature 546, 609–610 (2017)
V. Mittal, Epithelial mesenchymal transition in tumor metastasis. Annu. Rev. Pathol. 13, 395–412 (2018)
A. Dongre, R.A. Weinberg, New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 20, 69–84 (2019)
S. Lamouille, J. Xu, R. Derynck, Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 (2014)
D. Pei, X. Shu, A. Gassama-Diagne, J.P. Thiery, Mesenchymal-epithelial transition in development and reprogramming. Nat. Cell Biol. 21, 44–53 (2019)
Y. Sun, C. Li, J. Feng, Y. Li, X. Zhai, L. Zhang, C. Li, Ferritinophagic Flux Activation in CT26 Cells Contributed to EMT Inhibition Induced by a Novel Iron Chelator, DpdtpA. Oxid. Med. Cell. Longev. 2019, 1–14 (2019)
D. Guan, W. Zhou, H. Wei, T. Wang, K. Zheng, C. Yang, R. Feng, R. Xu, Y. Fu, C. Li, Y. Li, C. Li, Ferritinophagy-Mediated Ferroptosis and Activation of Keap1/Nrf2/HO-1 Pathway Were Conducive to EMT Inhibition of Gastric Cancer Cells in Action of 2,2’-Di-pyridineketone Hydrazone Dithiocarbamate Butyric Acid Ester. Oxid. Med. Cell. Longev. 2022, 3920664 (2022)
H. Li, W. Zhou, H. Wei, L. Li, X. Wang, Y. Li, S. Li, C. Li, Ferritinophagic Flux Was a Driving Force in Determination of Status of EMT, Ferroptosis, and NDRG1 Activation in Action of Mechanism of 2-Pyridylhydrazone Dithiocarbamate S-Acetic Acid. J. Oncol. 2021, 3015710 (2021)
J. Feng, C. Li, R. Xu, Y. Li, Q. Hou, R. Feng, S. Wang, L. Zhang, C. Li, DpdtC-Induced EMT inhibition in MGC-803 cells was partly through Ferritinophagy-Mediated ROS/p53 pathway. Oxid. Med. Cell. Longev. 2020, 9762390 (2020)
Z. Jiang, S.O. Lim, M. Yan, J.L. Hsu, J. Yao, Y. Wei, S.S. Chang, H. Yamaguchi, H.H. Lee, B. Ke, J.M. Hsu, L.C. Chan, G.N. Hortobagyi, L. Yang, C. Lin, D. Yu, M.C. Hung, TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J. Clin. Invest. 131, e139434 (2021)
W.S. Yang, R. SriRamaratnam, M.E. Welsch, K. Shimada, R. Skouta, V.S. Viswanathan, J.H. Cheah, P.A. Clemons, A.F. Shamji, C.B. Clish, L.M. Brown, A.W. Girotti, V.W. Cornish, S.L. Schreiber, B.R. Stockwell, Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014)
V.A.N. Kraft, C.T. Bezjian, S. Pfeiffer, L. Ringelstetter, C. Müller, F. Zandkarimi, J. Merl-Pham, X. Bao, N. Anastasov, J. Kössl, S. Brandner, J.D. Daniels, P. Schmitt-Kopplin, S.M. Hauck, B.R. Stockwell, K. Hadian, J.A. Schick, GTP Cyclohydrolase 1/Tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent. Sci. 6, 41–53 (2020)
M. Soula, R.A. Weber, O. Zilka, H. Alwaseem, K. La, F. Yen, H. Molina, J. Garcia-Bermudez, D.A. Pratt, K. Birsoy, Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 16, 1351–1360 (2020)
Q. Hu, W. Wei, D. Wu, F. Huang, M. Li, W. Li, J. Yin, Y. Peng, Y. Lu, Q. Zhao, L. Liu, Blockade of GCH1/BH4 Axis activates ferritinophagy to mitigate the resistance of colorectal cancer to erastin-induced ferroptosis. Front. Cell Dev. Biol. 10, 810327 (2022)
Y. Wang, M. Wang, H.X. Wu, R.H. Xu, Advancing to the era of cancer immunotherapy. Cancer Commun. (Lond) 41, 803–829 (2021)
L. Zhao, X. Zhou, F. Xie, L. Zhang, H. Yan, J. Huang, C. Zhang, F. Zhou, J. Chen, L. Zhang, Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. (Lond) 42, 88–116 (2022)
C. Xu, S. Sun, T. Johnson, R. Qi, S. Zhang, J. Zhang, K. Yang, The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 35, 109235 (2021)
E.P. Skaar, The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 6, e1000949 (2010)
R. Sottile, G. Federico, C. Garofalo, R. Tallerico, M.C. Faniello, B. Quaresima, C.M. Cristiani, M. Di Sanzo, G. Cuda, V. Ventura, A.K. Wagner, G. Contrò, N. Perrotti, E. Gulletta, S. Ferrone, K. Kärre, F.S. Costanzo, F. Carlomagno, E. Carbone, Iron and ferritin modulate MHC Class I expression and NK Cell recognition. Front. Immunol. 10, 224 (2019)
Y. Mou, J. Wu, Y. Zhang, O. Abdihamid, C. Duan, B. Li, Low expression of ferritinophagy-related NCOA4 gene in relation to unfavorable outcome and defective immune cells infiltration in clear cell renal carcinoma. BMC Cancer 21, 18 (2021)
T. Zuo, T. Fang, J. Zhang, J. Yang, R. Xu, Z. Wang, H. Deng, Q. Shen, pH-Sensitive molecular-switch-containing polymer nanoparticle for breast cancer therapy with ferritinophagy-cascade ferroptosis and tumor immune activation. Adv. Healthc. Mater. 10, e2100683 (2021)
W. Sun, J. Yan, H. Ma, J. Wu, Y. Zhang, Autophagy-dependent ferroptosis-related signature is closely associated with the prognosis and tumor immune escape of patients with glioma. Int. J. Gen. Med. 15, 253–270 (2022)
R. Kim, A. Hashimoto, N. Markosyan, V.A. Tyurin, Y.Y. Tyurina, G. Kar, S. Fu, M. Sehgal, L. Garcia-Gerique, A. Kossenkov, B.A. Gebregziabher, J.W. Tobias, K. Hicks, R.A. Halpin, N. Cvetesic, H. Deng, L. Donthireddy, A. Greenberg, B. Nam, R.H. Vonderheide, Y. Nefedova, V.E. Kagan, D.I. Gabrilovich, Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature 612, 338–346 (2022)
X. Ma, L. Xiao, L. Liu, L. Ye, P. Su, E. Bi, Q. Wang, M. Yang, J. Qian, Q. Yi, CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 33, 1001-1012 e1005 (2021)
N. Li, W. Wang, H. Zhou, Q. Wu, M. Duan, C. Liu, H. Wu, W. Deng, D. Shen, Q. Tang, Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic. Biol. Med. 160, 303–318 (2020)
M. Tang, Z. Huang, X. Luo, M. Liu, L. Wang, Z. Qi, S. Huang, J. Zhong, J.X. Chen, L. Li, D. Wu, L. Chen, Ferritinophagy activation and sideroflexin1-dependent mitochondria iron overload is involved in apelin-13-induced cardiomyocytes hypertrophy. Free Radic. Biol. Med. 134, 445–457 (2019)
T.T. Mai, A. Hamaï, A. Hienzsch, T. Cañeque, S. Müller, J. Wicinski, O. Cabaud, C. Leroy, A. David, V. Acevedo, A. Ryo, C. Ginestier, D. Birnbaum, E. Charafe-Jauffret, P. Codogno, M. Mehrpour, R. Rodriguez, Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat. Chem. 9, 1025–1033 (2017)
S. Ma, E.S. Henson, Y. Chen, S.B. Gibson, Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 7, e2307 (2016)
V. Trujillo-Alonso, E.C. Pratt, H. Zong, A. Lara-Martinez, C. Kaittanis, M.O. Rabie, V. Longo, M.W. Becker, G.J. Roboz, J. Grimm, M.L. Guzman, FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat. Nanotechnol. 14, 616–622 (2019)
M. Gryzik, M. Asperti, A. Denardo, P. Arosio, M. Poli, NCOA4-mediated ferritinophagy promotes ferroptosis induced by erastin, but not by RSL3 in HeLa cells. Biochim. Biophys. Acta Mol. Cell. Res. 1868, 118913 (2021)
G. Wei, M. Wang, T. Hyslop, Z. Wang, B.I. Carr, Vitamin K enhancement of sorafenib-mediated HCC cell growth inhibition in vitro and in vivo. Int. J. Cancer 127, 2949–2958 (2010)
W. Dong, Y. Tan, Q. Qin, B. Yang, Q. Zhu, L. Xu, Z. Liu, E. Song, Y. Song, Polybrominated diphenyl ethers quinone induces NCOA4-mediated ferritinophagy through selectively autophagic degradation of ferritin. Chem. Res. Toxicol. 32, 2509–2516 (2019)
T. Huang, Y. Sun, Y. Li, T. Wang, Y. Fu, C. Li, C. Li, Growth inhibition of a novel iron chelator, DpdtC, against hepatoma carcinoma cell lines partly attributed to ferritinophagy-mediated lysosomal ROS generation. Oxid. Med. Cell. Longev. 2018, 4928703 (2018)
S. Sui, J. Zhang, S. Xu, Q. Wang, P. Wang, D. Pang, Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells. Cell Death Dis. 10, 331 (2019)
N.D. Yang, S.H. Tan, S. Ng, Y. Shi, J. Zhou, K.S. Tan, W.S. Wong, H.M. Shen, Artesunate induces cell death in human cancer cells via enhancing lysosomal function and lysosomal degradation of ferritin. J. Biol. Chem. 289, 33425–33441 (2014)
Z.J. Li, H.Q. Dai, X.W. Huang, J. Feng, J.H. Deng, Z.X. Wang, X.M. Yang, Y.J. Liu, Y. Wu, P.H. Chen, H. Shi, J.G. Wang, J. Zhou, G.D. Lu, Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol. Sin. 42, 301–310 (2021)
S. Masaldan, S.A.S. Clatworthy, C. Gamell, P.M. Meggyesy, A.T. Rigopoulos, S. Haupt, Y. Haupt, D. Denoyer, P.A. Adlard, A.I. Bush, M.A. Cater, Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 14, 100–115 (2018)
J. Xiao, S. Zhang, B. Tu, X. Jiang, S. Cheng, Q. Tang, J. Zhang, X. Qin, B. Wang, Z. Zou, C. Chen, Arsenite induces ferroptosis in the neuronal cells via activation of ferritinophagy. Food Chem. Toxicol. 151, 2509–2516 (2021)
P.-L. Lin, H.-H. Tang, S.-Y. Wu, N.-S. Shaw, C.-L. Su, Saponin formosanin C-Induced ferritinophagy and ferroptosis in human hepatocellular carcinoma cells. Antioxidants 9, 682 (2020)
C. Zhao, D. Yu, Z. He, L. Bao, L. Feng, L. Chen, Z. Liu, X. Hu, N. Zhang, T. Wang, Y. Fu, Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic. Biol Med. 175, 236–248 (2021)
T. Oliveira, E. Hermann, D. Lin, W. Chowanadisai, E. Hull, M. Montgomery, HDAC inhibition induces EMT and alterations in cellular iron homeostasis to augment ferroptosis sensitivity in SW13 cells. Redox Biol. 47, 102149 (2021)
T.R. Daniels, T. Delgado, G. Helguera, M.L. Penichet, The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin. Immunol. 121, 159–176 (2006)
T.R. Daniels, T. Delgado, J.A. Rodriguez, G. Helguera, M.L. Penichet, The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 121, 144–158 (2006)
C.R. Chitambar, J.P. Wereley, Transferrin receptor-dependent and -independent iron transport in gallium-resistant human lymphoid leukemic cells. Blood 91, 4686–4693 (1998)
H. Yamanishi, S. Iyama, Y. Yamaguchi, Y. Kanakura, Y. Iwatani, Total iron-binding capacity calculated from serum transferrin concentration or serum iron concentration and unsaturated iron-binding capacity. Clin. Chem. 49, 175–178 (2003)
W.E. Ho, H.Y. Peh, T.K. Chan, W.S. Wong, Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol. Ther. 142, 126–139 (2014)
Z. Kong, R. Liu, Y. Cheng, Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomed. Pharmacother. 109, 2043–2053 (2019)
G.J. Anderson, D.M. Frazer, Current understanding of iron homeostasis. Am. J. Clin. Nutr. 106, 1559s–1566s (2017)
S.J. Dixon, D.N. Patel, M. Welsch, R. Skouta, E.D. Lee, M. Hayano, A.G. Thomas, C.E. Gleason, N.P. Tatonetti, B.S. Slusher, B.R. Stockwell, Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3, e02523 (2014)
D.L. Zhang, J. Wu, B.N. Shah, K.C. Greutélaers, M.C. Ghosh, H. Ollivierre, X.Z. Su, P.E. Thuma, G. Bedu-Addo, F.P. Mockenhaupt, V.R. Gordeuk, T.A. Rouault, Erythrocytic ferroportin reduces intracellular iron accumulation, hemolysis, and malaria risk. Science 359, 1520–1523 (2018)
A. Donovan, C.A. Lima, J.L. Pinkus, G.S. Pinkus, L.I. Zon, S. Robine, N.C. Andrews, The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 1, 191–200 (2005)
Z. Zhang, F. Zhang, P. An, X. Guo, Y. Shen, Y. Tao, Q. Wu, Y. Zhang, Y. Yu, B. Ning, G. Nie, M.D. Knutson, G.J. Anderson, F. Wang, Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood 118, 1912–1922 (2011)
Z. Zhang, F. Zhang, X. Guo, P. An, Y. Tao, F. Wang, Ferroportin1 in hepatocytes and macrophages is required for the efficient mobilization of body iron stores in mice. Hepatology 56, 961–971 (2012)
F. Wang, P.N. Paradkar, A.O. Custodio, D. McVey Ward, M.D. Fleming, D. Campagna, K.A. Roberts, V. Boyartchuk, W.F. Dietrich, J. Kaplan, N.C. Andrews, Genetic variation in Mon1a affects protein trafficking and modifies macrophage iron loading in mice. Nat. Genet. 39, 1025–1032 (2007)
H. Drakesmith, E. Nemeth, T. Ganz, Ironing out ferroportin. Cell Metab. 22, 777–787 (2015)
C.B. Billesbølle, C.M. Azumaya, R.C. Kretsch, A.S. Powers, S. Gonen, S. Schneider, T. Arvedson, R.O. Dror, Y. Cheng, A. Manglik, Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Nature 586, 807–811 (2020)
E. Nemeth, M.S. Tuttle, J. Powelson, M.B. Vaughn, A. Donovan, D.M. Ward, T. Ganz, J. Kaplan, Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 (2004)
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 82274153 and 82173846), Young Talent Lifting Project of China Association of Chinese Medicine [No. CACM-(2021-QNRC2-A08)], Shanghai Rising-Star Program (No.22QA1409100), 2021 Shanghai Science and Technology Innovation Action Plan (No. 21S11902800), Three-year Action Plan for Shanghai TCM Development and Inheritance Program [ZY(2021-2023)-0401, ZY(2021-2023)-0208], Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-D-202004), and Innovation team of high-level local universities in Shanghai: Strategic Innovation Team of TCM Chemical Biology.
The figures were created with BioRender software (BioRender.com).
Funding
This work was supported by National Natural Science Foundation of China (No. 82274153 and 82173846), Oriental Scholars of Shanghai Universities (TP2022081), Jiangxi Province Thousand Talents Program (jxsq2023102168), Young Talent Lifting Project of China Association of Chinese Medicine [No. CACM-(2021-QNRC2-A08)], Shanghai Rising-Star Program (No.22QA1409100), 2021 Shanghai Science and Technology Innovation Action Plan (No. 21S11902800), Three-year Action Plan for Shanghai TCM Development and Inheritance Program [ZY(2021–2023)-0401, ZY(2021–2023)-0208], Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-D-202004), and Innovation team of high-level local universities in Shanghai: Strategic Innovation Team of TCM Chemical Biology.
Author information
Authors and Affiliations
Contributions
Yi-Chen Liu and Yi-Ting Gong: wrote the draft manuscript. Qing-Yan Sun, Bei Wang, Yue Yan, and Yi-Xu Chen: helped with analysis and literature review. Li-Jun Zhang, Wei-Dong Zhang, and Xin Luan: designed and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, YC., Gong, YT., Sun, QY. et al. Ferritinophagy induced ferroptosis in the management of cancer. Cell Oncol. 47, 19–35 (2024). https://doi.org/10.1007/s13402-023-00858-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-023-00858-x