Abstract
Purpose
Pancreatic cancer is one of the most aggressive cancers. Preclinical and clinical data indicate that Notch 1 ligand jagged1 (JAG1) plays a pro-oncogenic role in several malignant cancers. As yet, however, the role of JAG1 in pancreatic cancer is poorly understood. The objective of the present study was to investigate JAG1 as a therapeutic target in human pancreatic cancer.
Methods
Expression levels of Notch signaling molecules were assessed using GEO datasets and Western blot analysis, respectively. Anti-tumor effects following JAG1 silencing were evaluated using in vitro and in vivo assays. Prognostic implications were assessed using GEO datasets.
Results
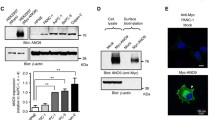
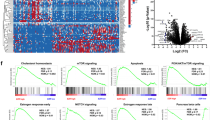
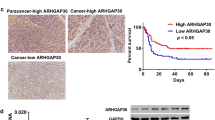
Using GEO datasets and Western blot analysis we detected significantly higher JAG1 mRNA and protein expression levels in pancreatic cancer compared to normal pancreatic tissues. JAG1 silencing significantly restrained the growth, migration and invasion of pancreatic cancer cells through the induction of apoptosis and blockade of various kinases independent of the Notch1 pathway. Combined JAG1 silencing and gemcitabine treatment showed synergistic anti-viability effects in human pancreatic cancer cells. JAG1 silencing also resulted in significant anti-cancer effects in vivo and high JAG1 expression was found to be associated with an adverse prognosis in pancreatic cancer patients.
Conclusions
From our data we conclude that JAG1 may be a promising therapeutic target in pancreatic cancer.






Similar content being viewed by others
References
R. Siegel, J. Ma, Z. Zou, A. Jemal, Cancer statistics, 2014. CA Cancer J Clin 64, 9–29 (2014)
J. Schmidt, U. Abel, J. Debus, S. Harig, K. Hoffmann, T. Herrmann, D. Bartsch, J. Klein, U. Mansmann, D. Jager, L. Capussotti, R. Kunz, M.W. Buchler, Open-label, multicenter, randomized phase iii trial of adjuvant chemoradiation plus interferon alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. Am J Clin Oncol 30, 4077–4083 (2012)
N. Bardeesy, R.A. DePinho, Pancreatic cancer biology and genetics. Nat Rev Cancer 2, 897–909 (2002)
C.E. Cano, Y. Motoo, J.L. Iovanna, Epithelial-to-mesenchymal transition in pancreatic adenocarcinoma. ScientificWorldJournal 10, 1947–1957 (2010)
D. Singh, G. Upadhyay, R.K. Srivastava, S. Shankar, Recent advances in pancreatic cancer: Biology, treatment, and prevention. Biochim Biophys Acta 1856, 13–27 (2015)
L. Miele, T. Golde, B. Osborne, Notch signaling in cancer. Curr Mol Med 6, 905–918 (2006)
S. Weijzen, P. Rizzo, M. Braid, R. Vaishnav, S.M. Jonkheer, A. Zlobin, B.A. Osborne, S. Gottipati, J.C. Aster, W.C. Hahn, M. Rudolf, K. Siziopikou, W.M. Kast, L. Miele, Activation of notch-1 signaling maintains the neoplastic phenotype in human ras-transformed cells. Nat Med 8, 979–986 (2002)
M. Reedijk, S. Odorcic, L. Chang, H. Zhang, N. Miller, D.R. McCready, G. Lockwood, S.E. Egan, High-level coexpression of jag1 and notch1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res 65, 8530–8537 (2005)
N. Sethi, X. Dai, C.G. Winter, Y. Kang, Tumor-derived jagged1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19, 192–205 (2011)
Y. Dai, G. Wilson, B. Huang, M. Peng, G. Teng, D. Zhang, R. Zhang, M.P. Ebert, J. Chen, B.C. Wong, K.W. Chan, J. George, L. Qiao, Silencing of jagged1 inhibits cell growth and invasion in colorectal cancer. Cell Death Dis 5, e1170 (2014)
B.W. Purow, R.M. Haque, M.W. Noel, Q. Su, M.J. Burdick, J. Lee, T. Sundaresan, S. Pastorino, J.K. Park, I. Mikolaenko, D. Maric, C.G. Eberhart, H.A. Fine, Expression of notch-1 andits ligands, delta-like-1 and jagged-1, is critical for glioma cell survival and proliferation. Cancer Res 65, 2353–2363 (2005)
J.T. Lin, M.K. Chen, K.T. Yeh, C.S. Chang, T.H. Chang, C.Y. Lin, Y.C. Wu, B.W. Su, K.D. Lee, P.J. Chang, Association of high levels of jagged-1 and notch-1 expression with poor prognosis in head and neck cancer. Ann Surg Oncol 17, 2976–2983 (2010)
S. Santagata, F. Demichelis, A. Riva, S. Varambally, M.D. Hofer, J.L. Kutok, R. Kim, J. Tang, J.E. Montie, A.M. Chinnaiyan, M.A. Rubin, J.C. Aster, Jagged1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res 64, 6854–6857 (2004)
J.H. Choi, J.T. Park, B. Davidson, P.J. Morin, M. Shih Ie, T.L. Wang, Jagged-1 and notch3 juxtacrine loop regulates ovarian tumor growth and adhesion. Cancer Res 68, 5716–5723 (2008)
F. Xing, A. Kobayashi, H. Okuda, M. Watabe, S.K. Pai, P.R. Pandey, S. Hirota, A. Wilber, Y.Y. Mo, B.E. Moore, W. Liu, K. Fukuda, M. Iiizumi, S. Sharma, Y. Liu, K. Wu, E. Peralta, K. Watabe, Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating notch signalling in brain. EMBO Mol Med 5, 384–396 (2013)
T.H. Cao, X. Ling, C. Chen, W. Tang, D.M. Hu, G.J. Yin, Role of mir-214-5p in the migration and invasion of pancreatic cancer cells. Eur Rev Med Pharmacol Sci 22, 7214–7221 (2018)
A. Kabashima-Niibe, H. Higuchi, H. Takaishi, Y. Masugi, Y. Matsuzaki, Y. Mabuchi, S. Funakoshi, M. Adachi, Y. Hamamoto, S. Kawachi, K. Aiura, Y. Kitagawa, M. Sakamoto, T. Hibi, Mesenchymal stem cells regulate epithelial-mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci 104, 157–164 (2013)
Z. Wang, Y. Li, D. Kong, S. Banerjee, A. Ahmad, A.S. Azmi, S. Ali, J.L. Abbruzzese, G.E. Gallick, F.H. Sarkar, Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res 69, 2400–2407 (2009)
J. Kleeff, M. Korc, M. Apte, C. La Vecchia, C.D. Johnson, A.V. Biankin, R.E. Neale, M. Tempero, D.A. Tuveson, R.H. Hruban, J.P. Neoptolemos, Pancreatic cancer. Nat Rev Dis Primers 2, 16022 (2016)
J. Lee, J. Hun Yun, J. Lee, C. Choi, J. Hoon Kim, Blockade of dual-specificity phosphatase 28 decreases chemo-resistance and migration in human pancreatic cancer cells. Sci Rep 5, 12296 (2015)
J. Lee, J. Lee, M. Kim, J.H. Kim, Fermented extraction of citrus unshiu peel inhibitsviability and migration of human pancreatic cancers. J Med Food 21, 5–12 (2018)
N. Radulovich, J.Y. Qian, M.S. Tsao, Human pancreatic duct epithelial cell model for kras transformation. Methods Enzymol 439, 1–13 (2008)
J. Lee, J. Lee, C. Choi, J.H. Kim, Blockade of integrin alpha3 attenuates human pancreatic cancer via inhibition of egfr signalling. Sci Rep 9, 2793 (2019)
J. Lee, J. Lee, J.H. Yun, C. Choi, S. Cho, S.J. Kim, J.H. Kim, Autocrine dusp28 signalingmediates pancreatic cancer malignancy via regulation of pdgf-a. Sci Rep 7, 12760 (2017)
J. Lee, D.S. Yang, S.I. Han, J.H. Yun, I.W. Kim, S.J. Kim, J.H. Kim, Aqueous extraction of citrus unshiu peel induces proangiogenic effects through the fak and erk1/2 signaling pathway in human umbilical vein endothelial cells. J Med Food 19, 569–577 (2016)
J. Lee, T. Ku, H. Yu, K. Chong, S.W. Ryu, K. Choi, C. Choi, Blockade of vegf-a suppresses tumor growth via inhibition of autocrine signaling through fak and akt. Cancer Lett 318, 221–225 (2012)
D.V. Zaykin, Optimally weighted z-test is a powerful method for combining probabilities in meta-analysis. J Evol Biol 24, 1836–1841 (2011)
P. Kurki, M. Vanderlaan, F. Dolbeare, J. Gray, E.M. Tan, Expression of proliferating cell nuclear antigen (pcna)/cyclin during the cell cycle. Exp Cell Res 166, 209–219 (1986)
G. Maga, U. Hubscher, Proliferating cell nuclear antigen (pcna): A dancer with many partners. J Cell Sci 116, 3051–3060 (2003)
P. Sidaway, Pancreatic cancer, new biomarkers improve standard screening. Nat Rev Clin Oncol 14, 262 (2017)
M. Momeny, F. Esmaeili, S. Hamzehlou, H. Yousefi, S. Javadikooshesh, V. Vahdatirad, Z. Alishahi, S.H. Mousavipak, D. Bashash, A.R. Dehpour, S.M. Tavangar, J. Tavakkoly-Bazzaz, P. Haddad, F. Kordbacheh, K. Alimoghaddam, A. Ghavamzadeh, S.H. Ghaffari, The erbb receptor inhibitor dacomitinib suppresses proliferation and invasion of pancreatic ductal adenocarcinoma cells. Cell Oncol 42, 491–504 (2019)
J. Zhang, L. Zhang, C. Li, C. Yang, L. Li, S. Song, H. Wu, F. Liu, L. Wang, J. Gu, Lox-1 is a poor prognostic indicator and induces epithelial-mesenchymal transition and metastasis in pancreatic cancer patients. Cell Oncol 41, 73–84 (2018)
J. Cordle, S. Johnson, J.Z. Tay, P. Roversi, M.B. Wilkin, B.H. de Madrid, H. Shimizu, S. Jensen, P. Whiteman, B. Jin, C. Redfield, M. Baron, S.M. Lea, P.A. Handford, A conserved face of the jagged/serrate dsl domain is involved in notch trans-activation and cis-inhibition. Nat Struct Mol Biol 15, 849–857 (2008)
C.R. Chillakuri, D. Sheppard, M.X. Ilagan, L.R. Holt, F. Abbott, S. Liang, R. Kopan, P.A. Handford, S.M. Lea, Structural analysis uncovers lipid-binding properties of notch ligands. Cell Rep 5, 861–867 (2013)
M.J. LaVoie, D.J. Selkoe, The notch ligands, jagged and delta, are sequentially processed by alpha-secretase and presenilin/gamma-secretase and release signaling fragments. J Biol Chem 278, 34427–34437 (2003)
S.F. Huang, Z.L. Yang, D.Q. Li, Z.Y. Liu, C.W. Wang, X.Y. Miao, Q. Zou, Y. Yuan, Jagged1 and dll4 expressions in benign and malignant pancreatic lesions and their clinicopathological significance. Hepatob Pancreat Dis 15, 640–646 (2016)
H.Y. Song, Y. Wang, H. Lan, Y.X. Zhang, Expression of notch receptors and their ligands in pancreatic ductal adenocarcinoma. Exp Ther Med 16, 53–60 (2018)
J. Dufraine, Y. Funahashi, J. Kitajewski, Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene 27, 5132–5137 (2008)
T. Kadesch, Notch signaling. The demise of elegant simplicity Curr Opin Genet Dev 14, 506–512 (2004)
F. Bocci, L. Gearhart-Serna, M. Boareto, M. Ribeiro, E. Ben-Jacob, G.R. Devi, H. Levine, J.N. Onuchic, M.K. Jolly, Toward understanding cancer stem cell heterogeneity in the tumor microenvironment. Proc Natl Acad Sci USA 116, 148–157 (2019)
H.M. Jeon, S.H. Kim, X. Jin, J.B. Park, S.H. Kim, K. Joshi, I. Nakano, H. Kim, Crosstalk between glioma-initiating cells and endothelial cells drives tumor progression. Cancer Res 74, 4482–4492 (2014)
T.S. Zhu, M.A. Costello, C.E. Talsma, C.G. Flack, J.G. Crowley, L.L. Hamm, X. He, S.L. Hervey-Jumper, J.A. Heth, K.M. Muraszko, F. DiMeco, A.L. Vescovi, X. Fan, Endothelial cells create a stem cell niche in glioblastoma by providing notch ligands that nurture self-renewal of cancer stem-like cells. Cancer Res 71, 6061–6071 (2011)
K. Imai, A. Takaoka, Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer 6, 714–727 (2006)
M. Metrich, A. Bezdek Pomey, C. Berthonneche, A. Sarre, M. Nemir, T. Pedrazzini, Jagged1 intracellular domain-mediated inhibition of notch1 signalling regulates cardiac homeostasis in the postnatal heart. Cardiovasc Res 108, 74–86 (2015)
Y. Hu, H. Su, X. Li, G. Guo, L. Cheng, R. Qin, G. Qing, H. Liu, The notch ligand jagged2 promotes pancreatic cancer metastasis independent of notch signaling activation. Mol Cancer Ther 14, 289–297 (2015)
J.D. Berlin, P. Catalano, J.P. Thomas, J.W. Kugler, D.G. Haller, A.B. Benson 3rd, Phase iii study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern cooperative oncology group trial e2297. Am J Clin Oncol 20, 3270–3275 (2002)
V. Heinemann, D. Quietzsch, F. Gieseler, M. Gonnermann, H. Schonekas, A. Rost, H. Neuhaus, C. Haag, M. Clemens, B. Heinrich, U. Vehling-Kaiser, M. Fuchs, D. Fleckenstein, W. Gesierich, D. Uthgenannt, H. Einsele, A. Holstege, A. Hinke, A. Schalhorn, R. Wilkowski, Randomized phase iii trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. Am J Clin Oncol 24, 3946–3952 (2006)
C. Louvet, R. Labianca, P. Hammel, G. Lledo, M.G. Zampino, T. Andre, A. Zaniboni, M. Ducreux, E. Aitini, J. Taieb, R. Faroux, C. Lepere, A. de Gramont, Gercor, Giscad, Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a gercor and giscad phase iii trial. Am J Clin Oncol 23, 3509–3516 (2005)
R. Herrmann, G. Bodoky, T. Ruhstaller, B. Glimelius, E. Bajetta, J. Schuller, P. Saletti, J. Bauer, A. Figer, B. Pestalozzi, C.H. Kohne, W. Mingrone, S.M. Stemmer, K. Tamas, G.V. Kornek, D. Koeberle, S. Cina, J. Bernhard, D. Dietrich, W. Scheithauer, Swiss Group for Clinical Cancer R, Central European Cooperative Oncology G, Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: A randomized, multicenter, phase iii trial of the swiss group for clinical cancer research and the central european cooperative oncology group. Am J Clin Oncol 25, 2212–2217 (2007)
M.J. Moore, D. Goldstein, J. Hamm, A. Figer, J.R. Hecht, S. Gallinger, H.J. Au, P. Murawa, D. Walde, R.A. Wolff, D. Campos, R. Lim, K. Ding, G. Clark, T. Voskoglou-Nomikos, M. Ptasynski, W. Parulekar, National Cancer Institute of Canada clinical trials G, Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase iii trial of the National Cancer Institute of Canada clinical trials group. Am J Clin Oncol 25, 1960–1966 (2007)
S. Cascinu, L. Verdecchia, N. Valeri, R. Berardi, M. Scartozzi, New target therapies in advanced pancreatic cancer. Ann Oncol 17, 148–152 (2006)
H.L. Kindler, G. Friberg, D.A. Singh, G. Locker, S. Nattam, M. Kozloff, D.A. Taber, T. Karrison, A. Dachman, W.M. Stadler, E.E. Vokes, Phase ii trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. Am J Clin Oncol 23, 8033–8040 (2005)
E. Aranda, J.L. Manzano, F. Rivera, M. Galan, M. Valladares-Ayerbes, C. Pericay, M.J. Safont, M.J. Mendez, A. Irigoyen, A. Arrivi, J. Sastre, E. Diaz-Rubio, Phase ii open-label study of erlotinib in combination with gemcitabine in unresectable and/or metastatic adenocarcinoma of the pancreas: Relationship between skin rash and survival (pantar study). AnnOncol 23, 1919–1925 (2012)
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03033899) and (2016R1A6A1A03012862).
Author information
Authors and Affiliations
Contributions
JW Lee conceived and supervised the experiments. JW Lee and JS Lee designed and performed the experiments. JS Lee performed the gene expression profiling and survival analyses. JW Lee, J Lee and JH Kim analyzed the data. JW Lee and JH Kim obtained the funding. JW Lee wrote and proofread the manuscript.
Corresponding authors
Ethics declarations
All experiments were approved by the Institutional Ethics Committee of the Jeju National University.
Conflict of interest
None declared.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, J., Lee, J. & Kim, J.H. Association of Jagged1 expression with malignancy and prognosis in human pancreatic cancer. Cell Oncol. 43, 821–834 (2020). https://doi.org/10.1007/s13402-020-00527-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00527-3