Abstract
Purpose
Ascofuranone is an antiviral antibiotic that is known to exert multiple anti-tumor effects, including cell cycle arrest, inhibition of mitochondrial respiration, and inhibition of angiogenesis. In this study, we investigated the molecular mechanisms underlying the anti-metastatic effects of ascofuranone in insulin-like growth factor-I (IGF-1)-responsive cancer cells.
Methods
The inhibitory effect of ascofuranone on cancer cell migration and invasion was assessed using scratch wound healing and Matrigel invasion assays, respectively. F-actin cytoskeleton organization was assessed using FITC conjugated phalloidin staining. Target gene expression was evaluated using Western blotting and gene silencing was performed using siRNA transfections. Finally, the anti-metastatic effect of ascofuranone was investigated in vivo.
Results
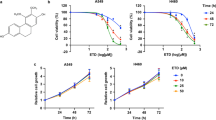
We found that ascofuranone suppressed IGF-1-induced cell migration, invasion and motility in multiple cancer cell lines. The effects of ascofuranone on actin cytoskeleton organization were found to be mediated by suppression of the mTOR/p70S6K/4EBP1 pathway. Ascofuranone inhibited IGF-1-induced mTOR phosphorylation and actin cytoskeleton organization via upregulation of AMPK and downregulation of Akt phosphorylation. It also selectively suppressed the IGF-1-induced mTOR complex (mTORC)1 by phosphorylation of Raptor, but did not affect mTORC2. Furthermore, we found that focal adhesion kinase (FAK) activation decreased in response to ascofuranone, rapamycin, compound C and wortmannin treatment. Finally, we found that ascofuranone suppressed phosphorylation of FAK and mTOR and dephosphorylation of Raptor in cancerous metastatic lung tissues in vivo.
Conclusions
Our data indicate that ascofuranone suppresses IGF-1-induced cancer cell migration and invasion by blocking actin cytoskeleton organization and FAK activation through inhibition of the mTORC1 pathway, and reveal a novel anti-metastatic function of this compound.








Similar content being viewed by others
References
Y. Wu, K. Zhang, J. Seong, J. Fan, S. Chien, Y. Wang, S. Lu, In-situ coupling between kinase activities and protein dynamics within single focal adhesions. Sci. Rep. 6, 29377 (2016)
D.J. Webb, K. Donais, L.A. Whitmore, S.M. Thomas, C.E. Turner, J.T. Parsons, A.F. Horwitz, FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6, 154–161 (2004)
A.M. Pasapera, I.C. Schneider, E. Rericha, D.D. Schlaepfer, C.M. Waterman, Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol. 188, 877–890 (2010)
M.B. Calalb, T.R. Polte, S.K. Hanks, Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 15, 954–963 (1995)
D.J. Sieg, C.R. Hauck, D. Ilic, C.K. Klingbeil, E. Schaefer, C.H. Damsky, D.D. Schlaepfer, FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2, 249–256 (2000)
H. Crossland, A.A. Kazi, C.H. Lang, J.A. Timmons, P. Pierre, D.J. Wilkinson, K. Smith, N.J. Szewczyk, P.J. Atherton, Focal adhesion kinase is required for IGF-I-mediated growth of skeletal muscle cells via a TSC2/mTOR/S6K1-associated pathway. Am. J. Physiol. Endocrinol. Metab. 305, E183–E193 (2013)
S.E. Dunn, M. Ehrlich, N.J. Sharp, K. Reiss, G. Solomon, R. Hawkins, R. Baserga, J.C. Barrett, A dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res. 58, 3353–3361 (1998)
J. Ma, H. Sawai, Y. Matsuo, N. Ochi, A. Yasuda, H. Takahashi, T. Wakasugi, H. Funahashi, M. Sato, H. Takeyama, IGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cells. J. Surg. Res. 160, 90–101 (2010)
Y. Zhou, S. Li, J. Li, D. Wang, Q. Li, Effect of microRNA-135a on cell proliferation, migration, invasion, apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt signaling pathway in non-small cell lung cancer. Cell. Physiol. Biochem. 42, 1431–1446 (2017)
M.A. Guvakova, E. Surmacz, The activated insulin-like growth factor I receptor induces depolarization in breast epithelial cells characterized by actin filament disassembly and tyrosine dephosphorylation of FAK, Cas, and paxillin. Exp. Cell Res. 251, 244–255 (1999)
L. Liu, L. Chen, J. Chung, S. Huang, Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene 27, 4998–5010 (2008)
R. Loewith, E. Jacinto, S. Wullschleger, A. Lorberg, J.L. Crespo, D. Bonenfant, W. Oppliger, P. Jenoe, M.N. Hall, Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10, 457–468 (2002)
D.H. Kim, D.D. Sarbassov, S.M. Ali, J.E. King, R.R. Latek, H. Erdjument-Bromage, P. Tempst, D.M. Sabatini, mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 (2002)
R.A. Saxton, D.M. Sabatini, mTOR signaling in growth, metabolism, and disease. Cell 169, 361–371 (2017)
P. Gulhati, K.A. Bowen, J. Liu, P.D. Stevens, P.G. Rychahou, M. Chen, E.Y. Lee, H.L. Weiss, K.L. O’Connor, T. Gao, B.M. Evers, mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 71, 3246–3256 (2011)
D.D. Sarbassov, S.M. Ali, D.H. Kim, D.A. Guertin, R.R. Latek, H. Erdjument-Bromage, P. Tempst, D.M. Sabatini, Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 (2004)
E. Jacinto, R. Loewith, A. Schmidt, S. Lin, M.A. Ruegg, A. Hall, M.N. Hall, Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6, 1122–1128 (2004)
L. Liu, Y. Luo, L. Chen, T. Shen, B. Xu, W. Chen, H. Zhou, X. Han, S. Huang, Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. J. Biol. Chem. 285, 38362–38373 (2010)
H. Sasaki, T. Hosokawa, M. Sawada, K. Ando, Isolation and structure of ascofuranone and ascofranol, antibiotics with hypolipidemic activity. J. Antibiot. 26, 676–680 (1973)
Y. Araki, T. Awakawa, M. Matsuzaki, R. Cho, Y. Matsuda, S. Hoshino, Y. Shinohara, M. Yamamoto, Y. Kido, D.K. Inaoka, K. Nagamune, K. Ito, I. Abe, K. Kita, Complete biosynthetic pathways of ascofuranone and ascochlorin in Acremonium egyptiacum. Proc. Natl. Acad. Sci. USA 116, 8269–8274 (2019)
H.J. Cho, J.H. Kang, J.Y. Kwak, T.S. Lee, I.S. Lee, N.G. Park, H. Nakajima, J. Magae, Y.C. Chang, Ascofuranone suppresses PMA-mediated matrix metalloproteinase-9 gene activation through the Ras/Raf/MEK/ERK- and Ap1-dependent mechanisms. Carcinogenesis 28, 1104–1110 (2007)
Y.J. Jeong, H.J. Cho, J. Magae, I.K. Lee, K.G. Park, Y.C. Chang, Ascofuranone suppresses EGF-induced HIF-1alpha protein synthesis by inhibition of the Akt/mTOR/p70S6K pathway in MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 273, 542–550 (2013)
J.H. Jeong, S.S. Kang, K.K. Park, H.W. Chang, J. Magae, Y.C. Chang, p53-independent induction of G1 arrest and p21WAF1/CIP1 expression by ascofuranone, an isoprenoid antibiotic, through downregulation of c-Myc. Mol. Cancer Ther. 9, 2102–2113 (2010)
H. Zhou, S. Huang, Role of mTOR signaling in tumor cell motility, invasion and metastasis. Curr. Protein Pept. Sci. 12, 30–42 (2011)
J. Xu, J. Ji, X.H. Yan, Cross-talk between AMPK and mTOR in regulating energy balance. Crit. Rev. Food Sci. Nutr. 52, 373–381 (2012)
F.Y. Lee, Y.Y. Zhen, C.M. Yuen, R. Fan, Y.T. Chen, J.J. Sheu, Y.L. Chen, C.J. Wang, C.K. Sun, H.K. Yip, The mTOR-FAK mechanotransduction signaling axis for focal adhesion maturation and cell proliferation. Am. J. Transl. Res. 9, 1603–1617 (2017)
B. Wang, X. Qi, D. Li, M. Feng, X. Meng, S. Fu, Expression of pY397 FAK promotes the development of non-small cell lung cancer. Oncol. Lett. 11, 979–983 (2016)
M.R. Pan, M.F. Hou, F. Ou-Yang, C.C. Wu, S.J. Chang, W.C. Hung, H.K. Yip, C.W. Luo, FAK is required for tumor metastasis-related fluid microenvironment in triple-negative breast cancer. J. Clin. Med. 8, E38, (2019). https://doi.org/10.3390/jcm8010038
S. Lamouille, J. Xu, R. Derynck, Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 15, 178–196 (2014)
X. Wang, P. Lai, Z. Zhang, M. Huang, L. Wang, M. Yin, D. Jin, R. Zhou, X. Bai, Targeted inhibition of mTORC2 prevents osteosarcoma cell migration and promotes apoptosis. Oncol. Rep. 32, 382–388 (2014)
S.L. Hwang, H.W. Chang, I.K. Lee, B.K. Yang, J. Magae, Y.C. Chang, Ascofuranone prevents ER stress-induced insulin resistance via activation of AMP-activated protein kinase in L6 myotube cells. Biochem. Biophys. Res. Commun. 396, 967–972 (2010)
K. Tsuji-Tamura, M. Ogawa, Inhibition of the PI3K-Akt and mTORC1 signaling pathways promotes the elongation of vascular endothelial cells. J. Cell Sci. 129, 1165–1178 (2016)
Henry W.S., Laszewski T., Tsang T., Beca F., Beck A.H., McAllister S.S., Toker A., Aspirin suppresses growth in PI3K-mutant breast cancer by activating AMPK and inhibiting mTORC1 signaling. Cancer Res. 77,790–801 (2017)
D.M. Gwinn, D.B. Shackelford, D.F. Egan, M.M. Mihaylova, A. Mery, D.S. Vasquez, B.E. Turk, R.J. Shaw, AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 (2008)
C.J. Potter, L.G. Pedraza, T. Xu, Akt regulates growth by directly phosphorylating Tsc2. Nat. Cell Biol. 4, 658–665 (2002)
M. Laplante, D.M. Sabatini, mTOR signaling in growth control and disease. Cell 149, 274–293 (2012)
B.D. Manning, A.R. Tee, M.N. Logsdon, J. Blenis, L.C. Cantley, Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell 10, 151–162 (2002)
K. Inoki, T. Zhu, K.L. Guan, TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 (2003)
J.P. Dales, S. Garcia, S. Meunier-Carpentier, L. Andrac-Meyer, O. Haddad, M.N. Lavaut, C. Allasia, P. Bonnier, C. Charpin, Overexpression of hypoxia-inducible factor HIF-1alpha predicts early relapse in breast cancer: retrospective study in a series of 745 patients. Int. J. Cancer 116, 734–739 (2005)
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) (NO. NRF-2020R1A2B5B01002648 and NO. NRF-2018R1C1B6008908).
Author information
Authors and Affiliations
Contributions
Yun-Jeong Jeong planned the overall experimental design and performed experiments. Soon-Kyung Hwang and Junji Magae contributed to the study design and provided scientific knowledge. Young-Chae Chang designed the research concept and supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Institutional Animal Care and Use Committee of the Catholic University of Daegu - DCIAFCR-181217-19-Y).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeong, YJ., Hwang, SK., Magae, J. et al. Ascofuranone suppresses invasion and F-actin cytoskeleton organization in cancer cells by inhibiting the mTOR complex 1 signaling pathway. Cell Oncol. 43, 793–805 (2020). https://doi.org/10.1007/s13402-020-00520-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00520-w