Abstract
Purpose
Several prognostic models have been developed to assess the efficacy and safety of sorafenib for metastatic renal cell carcinoma (mRCC), but few studies have validated its use in Chinese patients. The objective of this single center, single arm retrospective study was to examine the efficacy and safety of sorafenib and its related prognostic clinico-pathologic factors in Chinese mRCC patients.
Methods
One hundred thirty four mRCC patients were enrolled. All patients received 400 mg of sorafenib orally twice daily. The dose was subsequently adjusted in the event of treatment-induced toxicity. Tumor response, progression-free survival (PFS), overall survival (OS) and adverse events (AEs) were determined.
Results
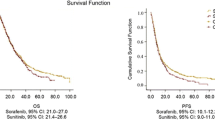
The median PFS and OS were 10 months (1–36 months) and 22 months (2–37 months), respectively. Complete, partial, and stable disease were observed in two (1.49 %), 24 (17.91 %), and 99 (73.88 %) patients, respectively. Hand/foot skin reactions, diarrhea and fatigue were the most commonly observed AEs following sorafenib treatment. Among the AEs, only 13 grades 3 and 4 were observed. Multivariate analysis revealed that independent predictive factors for PFS included Eastern Cooperative Oncology Group (ECOG) status, Memorial Sloan-Kettering Cancer Center (MSKCC) risk status, and bone metastasis (all p < 0.05). Factors associated with OS included MSKCC risk values, bone metastasis and sorafenib-induced hypertension (all p < 0.05).
Conclusion
The introduction of sorafenib therapy for mRCC in Chinese patients may lead to a favorable disease control with acceptable tolerability. In addition, the parameters predicting favorable outcomes, including ECOG status, MSKCC risk status and bone metastasis, may have prognostic value in clinical practice.


Similar content being viewed by others
References
B. Ljungberg, N.C. Cowan, D.C. Hanbury, M. Hora, M.A. Kuczyk, A.S. Merseburger, J.J. Patard, P.F. Mulders, I.C. Sinescu, European Association of Urology Guideline Group, EAU guidelines on renal cell carcinoma: the 2010 update. Eur. Urol. 58, 398–406 (2010)
C.L. Martel, P.N. Lara, Renal cell carcinoma: current status and future directions. Crit. Rev. Oncol. Hematol. 45, 177–190 (2003)
J. Bellmunt, M. Fishman, T. Eisen, D. Quinn, Expert opinion on the use of first-line sorafenib in selected metastatic renal cell carcinoma patients. Expert. Rev. Anticancer. Ther. 10, 825–835 (2010)
H.T. Cohen, F.J. McGovern, Renal-cell carcinoma. N. Engl. J. Med. 353, 2477–2490 (2005)
F. Rasmussen, Metastatic renal cell cancer. Cancer Imaging 13, 374–380 (2013)
B.I. Rini, Stabilization of disease in patients with metastatic renal cell carcinoma using sorafenib. Nat. Clin. Pract. Oncol. 3, 602–603 (2006)
R.J. Motzer, N.H. Bander, D.M. Nanus, Renal-cell carcinoma. N. Engl. J. Med. 335, 865–875 (1996)
K.T. Flaherty, Sorafenib in renal cell carcinoma. Clin. Cancer Res. 13, 747s–752s (2007)
M.J. Ratain, T. Eisen, W.M. Stadler, K.T. Flaherty, S.B. Kaye, G.L. Rosner, M. Gore, A.A. Desai, A. Patnaik, H.Q. Xiong, E. Rowinsky, J.L. Abbruzzese, C. Xia, R. Simantov, B. Schwartz, P.J. O’Dwyer, Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 24, 2505–2512 (2006)
B. Escudier, T. Eisen, W.M. Stadler, C. Szczylik, S. Oudard, M. Siebels, S. Negrier, C. Chevreau, E. Solska, A.A. Desai, F. Rolland, T. Demkow, T.E. Hutson, M. Gore, S. Freeman, B. Schwartz, M. Shan, R. Simantov, R.M. Bukowski, TARGET Study Group, Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 356, 125–134 (2007)
B. Escudier, C. Szczylik, T.E. Hutson, T. Demkow, M. Staehler, F. Rolland, S. Negrier, N. Laferriere, U.J. Scheuring, D. Cella, S. Shah, R.M. Bukowski, Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 27, 1280–1289 (2009)
C. Guevremont, C. Jeldres, P. Perrotte, P.I. Karakiewicz, Sorafenib in the management of metastatic renal cell carcinoma. Curr. Oncol. 16, S27–32 (2009)
G. Procopio, E. Verzoni, I. Testa, N. Nicolai, R. Salvioni, F. Debraud, Experience with sorafenib in the treatment of advanced renal cell carcinoma. Ther. Adv. Urol. 4, 303–313 (2012)
H. Zhang, B. Dong, J.J. Lu, X. Yao, S. Zhang, B. Dai, Y. Shen, Y. Zhu, D. Ye, Y. Huang, Efficacy of sorafenib on metastatic renal cell carcinoma in Asian patients: results from a multicenter study. BMC Cancer 9, 249 (2009)
H.S. Stafford, S.L. Saltzstein, S. Shimasaki, C. Sanders, T.M. Downs, G.R. Sadler, Racial/ethnic and gender disparities in renal cell carcinoma incidence and survival. J. Urol. 179, 1704–1708 (2008)
E.A. Eisenhauer, P. Therasse, J. Bogaerts, L.H. Schwartz, D. Sargent, R. Ford, J. Dancey, S. Arbuck, S. Gwyther, M. Mooney, L. Rubinstein, L. Shankar, L. Dodd, R. Kaplan, D. Lacombe, J. Verweij, New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009)
M.M. Oken, R.H. Creech, D.C. Tormey, J. Horton, T.E. Davis, E.T. McFadden, P.P. Carbone, Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 5, 649–655 (1982)
R.J. Motzer, M. Mazumdar, J. Bacik, W. Berg, A. Amsterdam, J. Ferrara, Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J. Clin. Oncol. 17, 2530–2540 (1999)
R.J. Motzer, T.E. Hutson, L. McCann, K. Deen, T.K. Choueiri, Overall survival in renal-cell carcinoma with pazopanib versus sunitinib(J). N. Engl. J. Med. 370, 1769–1770 (2014)
A. Mancuso, E.D. Di Paola, A. Leone, A. Catalano, F. Calabrò, L. Cerbone, A. Zivi, C. Messina, S. Alonso, L. Vigna, R. Caristo, C.N. Sternberg, Phase II escalation study of sorafenib in patients with metastatic renal cell carcinoma who have been previously treated with anti-angiogenic treatment. BJU Int. 109, 200–206 (2012)
B. Escudier, Sorafenib for the management of advanced renal cell carcinoma. Expert. Rev. Anticancer. Ther. 11, 825–836 (2011)
H. Akaza, T. Tsukamoto, M. Murai, K. Nakajima, S. Naito, Phase II study to investigate the efficacy, safety, and pharmacokinetics of sorafenib in Japanese patients with advanced renal cell carcinoma. Jpn. J. Clin. Oncol. 37, 755–762 (2007)
G. Procopio, E. Verzoni, S. Bracarda, S. Ricci, C. Sacco, L. Ridolfi, C. Porta, R. Miceli, N. Zilembo, E. Bajetta, Sorafenib with interleukin-2 vs sorafenib alone in metastatic renal cell carcinoma: the ROSORC trial. Br. J. Cancer 104, 1256–1261 (2011)
B.I. Rini, D.P. Cohen, D.R. Lu, I. Chen, S. Hariharan, M.E. Gore, R.A. Figlin, M.S. Baum, R.J. Motzer, Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J. Natl. Cancer Inst. 103, 763–773 (2011)
D.Y. Heng, W. Xie, M.M. Regan, M.A. Warren, A.R. Golshayan, C. Sahi, B.J. Eigl, J.D. Ruether, T. Cheng, S. North, P. Venner, J.J. Knox, K.N. Chi, C. Kollmannsberger, D.F. McDermott, W.K. Oh, M.B. Atkins, R.M. Bukowski, B.I. Rini, T.K. Choueiri, Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J. Clin. Oncol. 27, 5794–5799 (2009)
D.Y. Heng, W. Xie, M.M. Regan, L.C. Harshman, G.A. Bjarnason, U.N. Vaishampayan, M. Mackenzie, L. Wood, F. Donskov, M.H. Tan, S.Y. Rha, N. Agarwal, C. Kollmannsberger, B.I. Rini, T.K. Choueiri, External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 14, 141–148 (2013)
S. Patil, R.A. Figlin, T.E. Hutson, M.D. Michaelson, S. Négrier, S.T. Kim, X. Huang, R.J. Motzer, Prognostic factors for progression-free and overall survival with sunitinib targeted therapy and with cytokine as first-line therapy in patients with metastatic renal cell carcinoma. Ann. Oncol. 22, 295–300 (2011)
B.I. Rini, S.C. Campbell, B. Escudier, Renal cell carcinoma. Lancet 373, 1119–1132 (2009)
K. Nakano, K. Komatsu, T. Kubo, S. Natsui, A. Nukui, S. Kurokawa, M. Kobayashi, T. Morita, Hand-foot skin reaction is associated with the clinical outcome in patients with metastatic renal cell carcinoma treated with sorafenib. Jpn. J. Clin. Oncol. 43, 1023–1029 (2013)
A. Poprach, T. Pavlik, B. Melichar, I. Puzanov, L. Dusek, Z. Bortlicek, R. Vyzula, J. Abrahamova, T. Buchler, Czech Renal Cancer Cooperative Group, skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Ann. Oncol. 23(3137–3143) (2012)
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
mRCC patient survival in those with non-clear cell histology after sorafenib treatment. Kaplan-Meier curves of (A) progression-free survival (PFS) and (B) overall survival (OS) for 31 mRCC patients with non-clear-cell histology after sorafenib treatment. (GIF 20 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, Y., Cai, Y. et al. Efficacy of sorafenib correlates with Memorial Sloan-Kettering Cancer Center (MSKCC) risk classification and bone metastasis in Chinese patients with metastatic renal cell carcinoma. Cell Oncol. 39, 15–21 (2016). https://doi.org/10.1007/s13402-015-0245-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-015-0245-5