Abstract
Background
Previous research has suggested a putative utility of pancreatic (pro)enzymes in cancer treatment. The aim of the present study was to investigate the in vitro effects of a mixture of two pancreatic pro-enzymes, i.e., Chymotrypsinogen and Trypsinogen, and the enzyme Amylase on three human cancer cell lines, i.e., OE33 (derived from an oesophageal carcinoma), Panc1 (derived from a pancreatic carcinoma) and Caco-2 (derived from a colon carcinoma).
Results
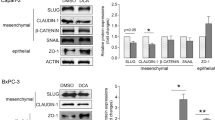
After treatment of the three cancer cell lines with different doses of the (pro)enzymes for up to 7 days, we observed (i) growth inhibition in a dose-dependent manner, (ii) enhanced expression of β-catenin and E-cadherin and decreased expression of several epithelial-mesenchymal transition (EMT)-associated genes, such as Vimentin, Snail and Slug, (iii) differentiation of Caco-2 cells, including the appearance of cell-specific differentiated structures such as microvilli and tight junctions, the acquisition of a more regular polygonal morphology, and an increased expression of the intestinal differentiation markers alkaline phosphatase and cytokeratin 8, and (iv) differentiation of Panc1 cells, including the formation of cell aggregates, an increment on lamellar bodies and an increased expression of the pancreatic differentiation markers glucagon and insulin.
Conclusions
Our results show that the treatment of three different human cancer cell lines with pancreatic (pro)enzymes results in an enhancement of cell adhesion, an attenuation of several EMT-associated markers, and an increase in the expression of several differentiation-associated markers, suggesting the acquisition of a less malignant phenotype and a decrease in proliferative capacity due to lineage-specific cellular differentiation.










Similar content being viewed by others
References
P.A. Andreasen, L. Kjoller, L. Christensen, M.J. Duffy, The urokinase-type plasminogen activator system in cancer metastasis: a review. Int. J. Cancer 72, 1–22 (1997)
J. Beard, The action of trypsin upon the living cells of jensen’s mouse-tumour: A preliminary note upon a research made (with a Grant from the Carnegie Trust). Br. Med. J. 1, 140–1 (1906)
R.W. Moss, An annotated bibliography of works by John Beard. Integr. Cancer Ther. 7, 317–21 (2008)
J. Beard, The cancer problem. Lancet 168, 281–283 (1905)
J.F. Novak, F. Trnka, Proenzyme therapy of cancer. Anticancer. Res. 25, 1157–77 (2005)
M. Wald, T. Olejar, V. Sebkova, M. Zadinova, M. Boubelik, P. Pouckova, Mixture of trypsin, chymotrypsin and papain reduces formation of metastases and extends survival time of C57Bl6 mice with syngeneic melanoma B16. Cancer Chemother. Pharmacol. 47, 16–22 (2001)
J. Beuth, B. Ost, A. Pakdaman, E. Rethfeldt, P.R. Bock, J. Hanisch et al., Impact of complementary oral enzyme application on the postoperative treatment results of breast cancer patients–results of an epidemiological multicentre retrolective cohort study. Cancer Chemother. Pharmacol. 47, 45–54 (2001)
T. Popiela, J. Kulig, J. Hanisch, P.R. Bock, Influence of a complementary treatment with oral enzymes on patients with colorectal cancers–an epidemiological retrolective cohort study. Cancer Chemother. Pharmacol. 47, 55–63 (2001)
A. Sakalova, P.R. Bock, L. Dedik, J. Hanisch, W. Schiess, S. Gazova et al., Retrolective cohort study of an additive therapy with an oral enzyme preparation in patients with multiple myeloma. Cancer Chemother Pharmacol 47, 38–44 (2001)
W.J. Nelson, Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem. Soc. Trans. 36, 149–55 (2008)
H. Peinado, D. Olmeda, A. Cano, Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer 7, 415–28 (2007)
R. Kemler, From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends. Genet. 93, 17–21 (1993)
M. Conacci-Sorrell, J. Zhurinsky, A. Ben-Ze’ev, The cadherin-catenin adhesion system in signaling and cancer. J. Clin. Invest. 109, 987–91 (2002)
B.M. Gumbiner, P.D. McCrea, Catenins as mediators of the cytoplasmic functions of cadherins. J. Cell Sci. Suppl. 17, 155–158 (1993)
B. Zhai, H.X. Yan, S.Q. Liu, L. Chen, M.C. Wu, H.Y. Wang, Reduced expression of P120 catenin in cholangiocarcinoma correlated with tumor clinicopathologic parameters. World J. Gastroenterol. 14, 3739–44 (2008)
E. Margineanu, C.E. Cotrutz, C. Cotrutz, Correlation between E-cadherin abnormal expressions in different types of cancer and the process of metastasis. Rev. Med. Chir. Soc. Med. Nat. Iasi. 112, 432–6 (2008)
I. Molina-Ortiz, R.A. Bartolome, P. Hernandez-Varas, G.P. Colo, J. Teixido, Overexpression of E-cadherin on melanoma cells inhibits chemokine-promoted invasion involving p190RhoGAP/p120ctn-dependent inactivation of RhoA. J. Biol. Chem. 284, 15147–57 (2009)
R.W. Pang, R.T. Poon, From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology 72, 30–44 (2007)
S. Tommasi, R. Pinto, B. Pilato, A. Paradiso, Molecular pathways and related target therapies in liver carcinoma. Curr. Pharm. Des. 13, 3279–87 (2007)
S. Boyault, D.S. Rickman, A. de Reynies, C. Balabaud, S. Rebouissou, E. Jeannot et al., Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 45, 42–52 (2007)
T. Parasassi, R. Brunelli, L. Bracci-Laudiero, G. Greco, A.C. Gustafsson, E.K. Krasnowska et al., Differentiation of normal and cancer cells induced by sulfhydryl reduction: biochemical and molecular mechanisms. Cell Death Differ. 12, 1285–96 (2005)
J.C. Fleet, L. Wang, O. Vitek, B.A. Craig, H.J. Edenberg, Gene expression profiling of Caco-2 BBe cells suggests a role for specific signaling pathways during intestinal differentiation. Physiol. Genomics 13, 57–68 (2003)
S. Sell, Stem cell origin of cancer and differentiation therapy. Crit. Rev. Oncol. Hematol. 51, 1–28 (2004)
R.L. Eckert, J.F. Crish, T. Efimova, S. Balasubramanian, Antioxidants regulate normal human keratinocyte differentiation. Biochem. Pharmacol. 68, 1125–31 (2004)
J. Leipner, R. Saller, Systemic enzyme therapy in oncology: Effect and mode of action. Drugs 59, 769–80 (2000)
E.R. Camp, V.J. Findlay, S.G. Vaena, J. Walsh, D.N. Lewin, D.P. Turner et al., Slug expression enhances tumor formation in a noninvasive rectal cancer model. J. Surg. Res. 170(1), 56–63 (2011)
P.B. Gupta, T.T. Onder, G. Jiang, K. Tao, C. Kuperwasser, R.A. Weinberg et al., Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138, 645–59 (2009)
A. Voulgari, A. Pintzas, Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim. Biophys. Acta 1796, 75–90 (2009)
J. Fogh, J.M. Fogh, T. Orfeo, One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 59, 221–6 (1977)
M. Pinto, S. Robineleon, M.D. Appay, M. Kedinger, N. Triadou, E. Dussaulx et al., Enterocyte-like differentiation and polarization of the human-colon carcinoma cell-line caco-2 in culture. Biol. Cell 47, 323–330 (1983)
M.D. Basson, G.A. Turowski, Z. Rashid, F. Hong, J.A. Madri, Regulation of human colonic cell line proliferation and phenotype by sodium butyrate. Dig. Dis. Sci. 41, 1989–93 (1996)
S.R. Wood, Q. Zhao, L.H. Smith, C.K. Daniels, Altered morphology in cultured rat intestinal epithelial IEC-6 cells is associated with alkaline phosphatase expression. Tissue Cell 35, 47–58 (2003)
S.K. Kurdistani, P. Arizti, C.L. Reimer, M.M. Sugrue, S.A. Aaronson, S.W. Lee, Inhibition of tumor cell growth by RTP/rit42 and its responsiveness to p53 and DNA damage. Cancer Res. 58, 4439–44 (1998)
J.P. Brunet, N. Jourdan, J. Cotte-Laffitte, C. Linxe, M. Geniteau-Legendre, A. Servin et al., Rotavirus infection induces cytoskeleton disorganization in human intestinal epithelial cells: implication of an increase in intracellular calcium concentration. J. Virol. 74, 10801–6 (2000)
R.J. Guang, J.L. Ford, Y.N. Fu, Y.Z. Li, L.M. Shaw, A.B. Pardee, Drg-1 as a differentiation-related, putative metastatic suppressor gene in human colon cancer. Cancer Res. 60, 749–755 (2000)
J.M. Anderson, Molecular structure of tight junctions and their role in epithelial transport. News Physiol. Sci. 16, 126–30 (2001)
Y. Kang, J. Massague, Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell 118, 277–9 (2004)
A.A. Hardikar, B. Marcus-Samuels, E. Geras-Raaka, B.M. Raaka, M.C. Gershengorn, Human pancreatic precursor cells secrete FGF2 to stimulate clustering into hormone-expressing islet-like cell aggregates. Proc. Natl. Acad. Sci. U. S. A. 100, 7117–22 (2003)
Y. Wu, J. Li, S. Saleem, S.P. Yee, A.A. Hardikar, R. Wang, c-Kit and stem cell factor regulate PANC-1 cell differentiation into insulin- and glucagon-producing cells. Lab. Invest. 90(1373–84) (2010)
J.A. Marchal, J. Prados, J. Campos, F. González, C. Melguizo, C. Velez et al., Therapeutic potential of differentiation in cancer and normal stem cells, in New Cell Differentiation Research Topics, ed. by H. Saitama (Nova Science Publisher, Inc, New York, 2008), pp. 7–77
J. Czyzewska, K. Guzinska-Ustymowicz, M. Ustymowicz, A. Pryczynicz, A. Kemona, The expression of E-cadherin-catenin complex in patients with advanced gastric cancer: role in formation of metastasis. Folia Histochem. Cytobiol. 48, 37–45 (2010)
I.J. Chalmers, M. Aubele, E. Hartmann, E. Braungart, M. Werner, H. Hofler, M.J. Atkinson, Mapping the chromosome 16 cadherin gene cluster to a minimal deleted region in ductal breast cancer. Cancer Genet. Cytogenet. 126, 39–44 (2001)
W. Feng, R. Orlandi, N. Zhao, M.L. Carcangiu, E. Tagliabue, J. Xu et al., Tumor suppressor genes are frequently methylated in lymph node metastases of breast cancers. B.M.C. Cancer 10(378–88) (2010)
M.T. Debies, D.R. Welch, Genetic basis of human breast cancer metastasis. J. Mammary Gland. Biol. Neoplasia 6, 441–51 (2001)
R.B. Hazan, R. Qiao, R. Keren, I. Badano, K. Suyama, Cadherin switch in tumor progression. Ann. N. Y. Acad. Sci. 1014, 155–63 (2004)
Y. Uchikado, H. Okumura, S. Ishigami, T. Setoyama, M. Matsumoto, T. Owaki et al., Increased Slug and decreased E-cadherin expression is related to poor prognosis in patients with gastric cancer. Gastric Cancer 14, 41–9 (2011)
Y. Li, C.Q. Chen, Y.L. He, S.R. Cai, D.J. Yang, W.L. He et al., Abnormal expression of E-cadherin in tumor cells is associated with poor prognosis of gastric carcinoma. J. Surg. Oncol. 106(3), 304–10 (2012)
X. Tian, Z. Liu, B. Niu, J. Zhang, T.K. Tan, S.R. Lee et al., E-cadherin/beta-catenin complex and the epithelial barrier. J. Biomed. Biotechnol. 2011, 1–6 (2011)
J.A. Marchal, M.C. Nunez, A. Aranega, M.A. Gallo, A. Espinosa, J.M. Campos, Acyclonucleosides, modified seco-nucleosides, and salicyl- or catechol-derived acyclic 5-fluorouracil O, N-acetals: antiproliferative activities, cellular differentiation and apoptosis. Curr. Med. Chem. 16, 1166–83 (2009)
J.P. Thiery, J.P. Sleeman, Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–42 (2006)
V. Bolos, H. Peinado, M.A. Perez-Moreno, M.F. Fraga, M. Esteller, A. Cano, The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell. Sci. 116, 499–511 (2003)
C. Castro Alves, E. Rosivatz, C. Schott, R. Hollweck, I. Becker et al., Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J. Pathol. 211, 507–15 (2007)
P. Jethwa, M. Naqvi, R.G. Hardy, N.A. Hotchin, S. Roberts, R. Spychal et al., Overexpression of Slug is associated with malignant progression of esophageal adenocarcinoma. World J. Gastroenterol. 14, 1044–52 (2008)
K. Zhang, D. Chen, X. Jiao, S. Zhang, X. Liu, J. Cao et al., Slug enhances invasion ability of pancreatic cancer cells through upregulation of matrix metalloproteinase-9 and actin cytoskeleton remodeling. Lab. Invest. 91, 426–38 (2011)
M.G. Mendez, S. Kojima, R.D. Goldman, Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 24, 1838–51 (2010)
Acknowledgments
Macarena Peran was supported by a grant from Jaen University, Spain (“Plan de Apoyo a la Investigación, Desarrollo Tecnológico e Innovación. IV. Programa de Ayudas a los Investigadores”). Work in the laboratory of Bath University was funded by Propanc Pty Ltd. Work in the laboratory of J.A. Marchal at Granada University was funded by the Instituto de Salud Carlos III (Fondo de Investigación Sanitaria, FEDER, grant number PI10/02295).
Conflict of interest
Dr Julian Kenyon is the Founder and Scientific Director of Propanc Pty Ltd and owns stock in the company. Propanc Pty Ltd and the University of Bath have filed a joint patent from the scientific research undertaken in this report
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Perán, M., Marchal, J.A., García, M.A. et al. In vitro treatment of carcinoma cell lines with pancreatic (pro)enzymes suppresses the EMT programme and promotes cell differentiation. Cell Oncol. 36, 289–301 (2013). https://doi.org/10.1007/s13402-013-0134-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-013-0134-8