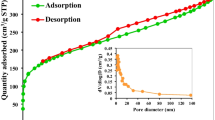
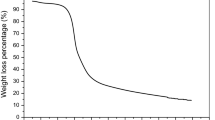
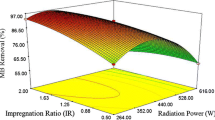
Abstract
This study employed palm date stone (PDS) as an alternative precursor to produce activated carbon (PDSAC) via microwave-assisted ZnCl2 activation. The Box-Behnken design (BBD) method was utilized to optimize the critical adsorption factors for removal of crystal violet dye (CV) dye by PDSAC. The optimized adsorption parameters were obtained; A: PDSAC dose (0.02–0.1 g/100 mL), B: pH (4–10), and C: contact time (20–200 min). Moreover, the numerical desirability function approach was adopted to statistically validate the output of BBD results and to estimate the best operational adsorption conditions. The dye adsorption kinetics were well described by the pseudo-second-order (PSO) model. Moreover, the Freundlich isotherm model is the best model to describe the heterogeneous nature of the adsorption process of CV by PDSAC. Thus, the maximum adsorption capacity (qmax) of PDSAC for the CV dye was 33.7 mg/g at 25 °C. The adsorption mechanism of CV by PDSAC can be assigned to different types of physical and chemical contributions such as pore filling, H-bonding, electrostatic forces, and π-π stacking interaction. Hence, this study introduces PDS as a renewable precursor for producing activated carbon with potential application for toxic dye removal from aqueous media.










Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ashrafi M, Bagherian G, Arab Chamjangali M, Goudarzi N (2018) Removal of brilliant green and crystal violet from mono-and bi-component aqueous solutions using NaOH-modified walnut shell. Anal Bioanal Chem Res 5(1):95–114. https://doi.org/10.22036/ABCR.2018.106139.1172
Mohanty S, Moulick S, Maji SK (2020) Adsorption/photodegradation of crystal violet (basic dye) from aqueous solution by hydrothermally synthesized titanate nanotube (TNT). J Water Process Eng 37:101428. https://doi.org/10.1016/j.jwpe.2020.101428
Ciğeroğlu Z, Kazan-Kaya ES, El Messaoudi N, Fernine Y, Américo-Pinheiro JH, Jada A (2023) Remediation of tetracycline from aqueous solution through adsorption on g-C3N4-ZnO-BaTiO3 nanocomposite: optimization, modeling, and theoretical calculation. J Mol Liq 369:120866. https://doi.org/10.1016/j.molliq.2022.120866
Zhao H, Zhong H, Jiang Y, Li H, Tang P, Li D, Feng Y (2022) Porous ZnCl2-activated carbon from shaddock peel: methylene blue adsorption behavior. Materials 15(3):895. https://doi.org/10.3390/ma15030895
Ihaddaden S, Aberkane D, Boukerroui A, Robert D (2022) Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia ficus indica). J Water Process Eng 49:102952. https://doi.org/10.1016/j.jwpe.2022.102952
Zaharia M, Vasiliu A, Trofin M, Pamfil D, Bucătariu F, Racoviţă S, Mihai M (2021) Design of multifunctional composite materials based on acrylic ion exchangers and CaCO3 as sorbents for small organic molecules. React Funct Polym 166:104997. https://doi.org/10.1016/j.reactfunctpolym.2021.104997
De Toledo W, Pinheiro RA, Trava-Airoldi V, Corat EJ (2022) Development of boron-doped diamond (BDD) deposited on carbon nanotubes (CNT) to form BDD/CNT structures relevant for electrochemical degradation. Diam Relat Mater 127:109159. https://doi.org/10.1016/j.diamond.2022.109159
El Messaoudi N, Ciğeroğlu Z, Şenol ZM, Kazan-Kaya ES, Fernine Y, Gubernat S, Lopicic Z (2024) Green synthesis of CuFe2O4 nanoparticles from bioresource extracts and their applications in different areas: a review. Biomass Convers Biorefinery 6:1–22. https://doi.org/10.1007/s13399-023-05264-9
Dinh VC, Hou CH, Dao TN (2022) O, N-doped porous biochar by air oxidation for enhancing heavy metal removal: the role of O, N functional groups. Chemosphere 293:133622. https://doi.org/10.1016/j.chemosphere.2022.133622
Azam K, Shezad N, Shafiq I, Akhter P, Akhtar F, Jamil F, Shafique S, Park Y, Hussain M (2022) A review on activated carbon modifications for the treatment of wastewater containing anionic dyes. Chemosphere 306:135566. https://doi.org/10.1016/j.chemosphere.2022.135566
Dao MU, Le HS, Hoang HY, Tran VA, Doan V, Le TTN, Sirotkin A, Le VT (2021) Natural core-shell structure activated carbon beads derived from Litsea glutinosa seeds for removal of methylene blue: Facile preparation, characterization, and adsorption properties. Environ Res 198:110481. https://doi.org/10.1016/j.envres.2020.110481
Zhang G, Lei B, Chen S, Xie H, Zhou G (2021) Activated carbon adsorbents with micro-mesoporous structure derived from waste biomass by stepwise activation for toluene removal from air. J Environ Chem Eng 9(4):105387. https://doi.org/10.1016/j.jece.2021.105387
Lewoyehu M (2021) Comprehensive review on synthesis and application of activated carbon from agricultural residues for the remediation of venomous pollutants in wastewater. J Anal Appl Pyrol 159:105279. https://doi.org/10.1016/j.jaap.2021.105279
Karri RR, Sahu J, Jayakumar N (2017) Optimal isotherm parameters for phenol adsorption from aqueous solutions onto coconut shell based activated carbon: error analysis of linear and non-linear methods. J Taiwan Inst Chem Eng 80:472–487. https://doi.org/10.1016/j.jtice.2017.08.004
Karri RR, Sahu J, Meikap B (2020) Improving efficacy of Cr (VI) adsorption process on sustainable adsorbent derived from waste biomass (sugarcane bagasse) with help of ant colony optimization. Ind Crops Prod 143:111927. https://doi.org/10.1016/j.indcrop.2019.111927
Sahu J, Karri RR, Jayakumar N (2021) Improvement in phenol adsorption capacity on eco-friendly biosorbent derived from waste palm-oil shells using optimized parametric modelling of isotherms and kinetics by differential evolution. Ind Crops Prod 164:113333. https://doi.org/10.1016/j.indcrop.2021.113333
Peighambardoust SJ, Foroutan R, Khatooni H, Ramavandi B (2021) Decoration of Citrus limon wood carbon with Fe3O4 to enhanced Cd2+ removal: a reclaimable and magnetic nanocomposite. Chemosphere 282:131088. https://doi.org/10.1016/j.chemosphere.2021.131088
Foroutan R, Mohammadi R, Ramavandi B, Bastanian M (2018) Removal characteristics of chromium by activated carbon/CoFe2O4 magnetic composite and Phoenix dactylifera stone carbon. Korean J Chem Eng 35(11):2207–2219. https://doi.org/10.1007/s11814-018-0145-2
Mehmandost N, Goudarzi N, Chamjangali MA, Bagherian G (2023) Application of chemometrics tools for removal of crystal violet and methylene blue in binary solution by eco-friendly magnetic adsorbent modified on Heracleum persicum waste. Spectrochim Acta Part A Mol Biomol Spectrosc 292:122415. https://doi.org/10.1016/j.saa.2023.122415
Tu B, Chen H, Xue S, Deng J, Tao H (2021) Ultrafast and efficient removal of aqueous Cr (VI) using iron oxide nanoparticles supported on Bermuda grass-based activated carbon. J Mol Liq 334:116026. https://doi.org/10.1016/j.molliq.2021.116026
Arumugasamy SK, Chellasamy G, Lee S, Govindaraju S, Yun K (2022) TriMOF synergized on the surface of activated carbon produced from pineapple leaves for the environmental pollutant reduction and oxygen evolution process. Chemosphere 286:131893. https://doi.org/10.1016/j.chemosphere.2021.131893
Safinejad A, Goudarzi N, Chamjangali MA, Bagherian G (2017) Effective simultaneous removal of Pb (II) and Cd (II) ions by a new magnetic zeolite prepared from stem sweep. Mater Res Express 4(11):116104. https://doi.org/10.1088/2053-1591/aa9738
Saadi W, Rodríguez-Sánchez S, Ruiz B, Najar-Souissi S, Ouederni A, Fuente E (2022) From pomegranate peels waste to one-step alkaline carbonate activated carbons. Prospect as sustainable adsorbent for the renewable energy production. J Environ Chem Eng 10(1):107010. https://doi.org/10.1016/j.jece.2021.107010
Alharbi HA, Hameed BH, Alotaibi KD, Al-Oud SS, Al-Modaihsh AS (2022) Recent methods in the production of activated carbon from date palm residues for the adsorption of textile dyes: a review. Front Environ Sci 10:996953. https://doi.org/10.3389/fenvs.2022.996953
Bensidhom G, Hassen-Trabelsi AB, Alper K, Sghairoun M, Zaafouri K, Trabelsi I (2018) Pyrolysis of date palm waste in a fixed-bed reactor: characterization of pyrolytic products. Biores Technol 247:363–369. https://doi.org/10.1016/j.biortech.2017.09.066
Chao CT, Krueger RR (2007) The date palm (Phoenix dactylifera L.): overview of biology, uses, and cultivation. HortScience 42(5):1077–82. https://doi.org/10.21273/HORTSCI.42.5.1077
Cuong DV, Hou CH (2024) Enhancing phosphorus removal through layered double hydroxide-decorated biochars: unveiling pore structure and surface functionalization. J Taiwan Inst Chem Eng 155:105273. https://doi.org/10.1016/j.jtice.2023.105273
Hijab M, Parthasarathy P, Mackey HR, Al-Ansari T, McKay G (2021) Minimizing adsorbent requirements using multi-stage batch adsorption for malachite green removal using microwave date-stone activated carbons. Chem Eng Process-Process Intensif 167:108318. https://doi.org/10.1016/j.cep.2021.108318
Khomri ME, Messaoudi NE, Dbik A, Bentahar S, Fernine Y, Bouich A, Lacherai A, Jada A (2022) Modification of low-cost adsorbent prepared from agricultural solid waste for the adsorption and desorption of cationic dye. Emergent Mater 5(6):1679–88. https://doi.org/10.1007/s42247-022-00390-y
Al-Qaessi F (2010) Production of activated carbon from date stones by using zinc chloride. Energy Sources, Part A: Recover, Utilization, Environ Effects 32(10):917–930. https://doi.org/10.1080/15567030903493062
Sirajo L, Zaini MAA (2022) Adsorption of water pollutants using H3PO4-activated lignocellulosic agricultural waste: a mini review. Toxin Rev 42(1):349–361. https://doi.org/10.1080/15569543.2022.2062775
Genli N, Kutluay S, Baytar O, ŞahiN Ö (2021) Preparation and characterization of activated carbon from hydrochar by hydrothermal carbonization of chickpea stem: an application in methylene blue removal by RSM optimization. Int J Phytoremediation 24(1):88–100. https://doi.org/10.1080/15226514.2021.1926911
Yağmur HK, Kaya İ (2021) Synthesis and characterization of magnetic ZnCl2-activated carbon produced from coconut shell for the adsorption of methylene blue. J Mol Struct 1232:130071. https://doi.org/10.1016/j.molstruc.2021.130071
Luo X, Cai Y, Li L, Zeng J (2019) Cr(VI) adsorption performance and mechanism of an effective activated carbon prepared from bagasse with a one-step pyrolysis and ZnCl2 activation method. Cellulose 26(8):4921–4934. https://doi.org/10.1007/s10570-019-02418-9
Nassar H, Zyoud A, El-Hamouz A, Tanbour R, Halayqa N, Hilal HS (2020) Aqueous nitrate ion adsorption/desorption by olive solid waste-based carbon activated using ZnCl2. Sustain Chem Pharm 18:100335. https://doi.org/10.1016/j.scp.2020.100335
Ramasamy SP, Jayabalakrishnan RM, Maheswari M, Boomiraj K, Oumabady S (2021) Coconut shell derived ZnCl2 activated carbon for malachite green dye removal. Water Sci Technol 83(5):1167–1182. https://doi.org/10.2166/wst.2021.050
Mu’azu ND, Zubair M, Jarrah N, Alagha O, Al-Harthi MA, Essa MH (2020) Sewage sludge ZnCl2-activated carbon intercalated MgFe–LDH nanocomposites: insight of the sorption mechanism of improved removal of phenol from water. Int J Mol Sci 21(5):1563. https://doi.org/10.3390/ijms21051563
Lee LZ, MaA Z (2020) One-step ZnCl2/FeCl3 composites preparation of magnetic activated carbon for effective adsorption of rhodamine B dye. Toxin Rev 41(1):64–81. https://doi.org/10.1080/15569543.2020.1837172
Zhao W, Cui Y, Zhou S, Ye J, Sun J, Liu X (2022) Rapid adsorption of dyes from aqueous solutions by modified lignin derived superparamagnetic composites. J Mol Struct 1261:132954. https://doi.org/10.1016/j.molstruc.2022.132954
Madankar CS, Bhagwat SS, Meshram PD (2021) Cd2+ removal from synthetic waters by ZnCl2-activated carbon. Mater Today: Proc 45:4684–4688. https://doi.org/10.1016/j.matpr.2021.01.118
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: optimization, reusability, kinetic and equilibrium studies. J Magn Magn Mater 404:179–189. https://doi.org/10.1016/j.jmmm.2015.12.040
Sing K (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57(4):603–619. https://doi.org/10.1351/pac198557040603
Kaur B, Gupta RK, Bhunia H (2019) Chemically activated nanoporous carbon adsorbents from waste plastic for CO2 capture: breakthrough adsorption study. Microporous Mesoporous Mater 282:146–158. https://doi.org/10.1016/j.micromeso.2019.03.025
Poletto M, Ornaghi HL, Zattera AJ (2014) Native cellulose: structure, characterization and thermal properties. Materials 7(9):6105–6119. https://doi.org/10.3390/ma7096105
Basheer AO, Hanafiah MM, AlSaadi MA, Al-Douri Y, Malek MA, Aljumaily MM, Fiyadh SS (2019) Synthesis and characterization of natural extracted precursor Date palm fibre-based activated carbon for aluminum removal by RSM optimization. Processes 7(5):249. https://doi.org/10.3390/pr7050249
Hadi S, Taheri E, Amin MM, Fatehizadeh A, Lima ÉC (2020) Fabrication of activated carbon from pomegranate husk by dual consecutive chemical activation for 4-chlorophenol adsorption. Environ Sci Pollut Res 28(11):13919–13930. https://doi.org/10.1007/s11356-020-11624-z
Bumajdad A, Hasila P (2023) Surface modification of date palm activated carbonaceous materials for heavy metal removal and CO2 adsorption. Arab J Chem 16(1):104403. https://doi.org/10.1016/j.arabjc.2022.104403
Ahmad MA, Eusoff MA, Oladoye PO, Adegoke KA, Bello OS (2020) Statistical optimization of Remazol Brilliant Blue R dye adsorption onto activated carbon prepared from pomegranate fruit peel. Chem Data Collect 28:100426. https://doi.org/10.1016/j.cdc.2020.100426
Jawad AH, Abdulhameed AS, Wilson LD, Hanafiah Ma KM, Nawawi W, ALOthman ZA, Khan MR (2021) Fabrication of Schiff’s base chitosan-glutaraldehyde/activated charcoal composite for cationic dye removal: optimization using response surface methodology. J Polym Environ 29(9):2855–2868. https://doi.org/10.1007/s10924-021-02057-x
Muthusaravanan S, Balasubramani K, Suresh R, Ganesh RS, Sivarajasekar N, Arul H, Rambabu K, Bharath G, Sathishkumar VE, Murthy AP, Banat F (2021) Adsorptive removal of noxious atrazine using graphene oxide nanosheets: insights to process optimization, equilibrium, kinetics, and density functional theory calculations. Environ Res 200:111428. https://doi.org/10.1016/j.envres.2021.111428
Jawad AH, Rangabhashiyam S, Abdulhameed AS, Syed-Hassan SSA, ALOthman ZA, Wilson LD (2022) Process optimization and adsorptive mechanism for reactive Blue 19 dye by magnetic crosslinked chitosan/MGO/Fe3O4 biocomposite. J Polym Environ 30(7):2759–2773. https://doi.org/10.1007/s10924-022-02382-9
Baig U, Uddin MK, Gondal M (2020) Removal of hazardous azo dye from water using synthetic nano adsorbent: facile synthesis, characterization, adsorption, regeneration, and design of experiments. Colloids Surf, A 584:124031. https://doi.org/10.1016/j.colsurfa.2019.124031
Lagergren S (1907) Zur Theorie der sogenannten Adsorption gelöster Stoffe. Z Chem Ind Kolloide 2(1):15. https://doi.org/10.1007/bf01501332
Ho Y, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70(2):115–124. https://doi.org/10.1016/s0923-0467(98)00076-1
Lingamdinne LP, Godlaveeti SK, Angaru GKR, Chang Y, Nagireddy RR, Somala AR, Koduru JR (2022) Highly efficient surface sequestration of Pb2+ and Cr3+ from water using a Mn3O4 anchored reduced graphene oxide: selective removal of Pb2+ from real water. Chemosphere 299:134457. https://doi.org/10.1016/j.chemosphere.2022.134457
El Khomri M, El Messaoudi N, Dbik A, Bentahar S, Lacherai A, Chegini ZG, Bouich A (2021) Removal of Congo red from aqueous solution in single and binary mixture systems using Argan nutshell wood. Pigm Resin Technol 51(5):477–488. https://doi.org/10.1108/PRT-04-2021-0045
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:327–356
Chandrasekaran M, Sivakumar VM, Thirumarimurugan M (2016) Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent. J Taiwan Inst Chem Eng 63:354–362. https://doi.org/10.1016/j.jtice.2016.03.034
Yusuff AS, Ajayi O, Popoola LT (2021) Application of Taguchi design approach to parametric optimization of adsorption of crystal violet dye by activated carbon from poultry litter. Sci Afr 13:e00850. https://doi.org/10.1016/j.sciaf.2021.e00850
Abdel-Salam AH, Ewais HA, Basaleh AS (2017) Silver nanoparticles immobilised on the activated carbon as efficient adsorbent for removal of crystal violet dye from aqueous solutions. A kinetic study. J Mol Liq 248:833–841. https://doi.org/10.1016/j.molliq.2017.10.109
Dil EA, Ghaedi M, Ghaedi A, Asfaram A, Jamshidi M, Purkait MK (2016) Application of artificial neural network and response surface methodology for the removal of crystal violet by zinc oxide nanorods loaded on activate carbon: kinetics and equilibrium study. J Taiwan Inst Chem Eng 59:210–220. https://doi.org/10.1016/j.jtice.2015.07.023
Zhao Y, Zhao Z, Yang X, Ren Z (2021) Xanthate modified magnetic activated carbon for efficient removal of cationic dyes and tetracycline hydrochloride from aqueous solutions. Colloids Surf, A 615:126273. https://doi.org/10.1016/j.colsurfa.2021.126273
Mohanty K, Naidu JT, Meikap ABC, Biswas MN (2006) Removal of crystal violet from wastewater by activated carbons prepared from rice husk. Ind Eng Chem Res 45(14):5165–5171. https://doi.org/10.1021/ie060257r
Wathukarage A, Herath I, Iqbal MCM, Vithanage M (2017) Mechanistic understanding of crystal violet dye sorption by woody biochar: implications for wastewater treatment. Environ Geochem Health 41(4):1647–1661. https://doi.org/10.1007/s10653-017-0013-8
Kashi E, Surip SN, Khadiran T, Nawawi W, De Luna Y, Yaseen ZM, Jawad AH (2024) High adsorptive performance of chitosan-microalgae-carbon-doped TiO2 (kronos)/ salicylaldehyde for brilliant green dye adsorption: Optimization and mechanistic approach. Int J Biol Macromol 259:129147. https://doi.org/10.1016/j.ijbiomac.2023.129147
Alcalde-Garcia F, Prasher SO, Kaliaguine S, Tavares JR, Dumont M (2023) Desorption strategies and reusability of biopolymeric adsorbents and semisynthetic derivatives in hydrogel and hydrogel composites used in adsorption processes. ACS Eng Au 3(6):443–460. https://doi.org/10.1021/acsengineeringau.3c00022
Xing W, Liu Q, Wang J, Xia S, Ma L, Lu R, Zhang Y, Huang Y, Wu G (2021) High selectivity and reusability of biomass-based adsorbent for chloramphenicol removal. Nanomaterials 11:2950. https://doi.org/10.3390/nano11112950
Acknowledgements
The authors are thankful to the Faculty of Applied Sciences, Universiti Teknologi MARA (UiTM) Shah Alam, Malaysia for the research facilities. The author Ahmad Hapiz is thankful to the Government of West Nusa Tenggara (NTB) Province, Indonesia, as well as the Education Development Institute (LPP) NTB and the Regional Research and Innovation Agency (BRIDA) NTB for providing a fully funded scholarship. The author (Zeid A. ALOthman) is grateful to the Researchers Supporting Project No. (RSP2024R1), King Saud University, Riyadh, Saudi Arabia.
Funding
The author Ahmad Hapiz is thankful to the Government of West Nusa Tenggara (NTB) Province, Indonesia, as well as the Education Development Institute (LPP) NTB and the Regional Research and Innovation Agency (BRIDA) NTB for providing a fully funded scholarship. The author (Zeid A. ALOthman) is grateful to the Researchers Supporting Project No. (RSP2024R1), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Nurul Adila Alia Che Hassan: formal analysis, validation, data curation. Ahmad Hapiz: formal analysis, validation, data curation, methodology, software, writing—original. Ali H. Jawad: conceptualization, resources, visualization, supervision, project administration, writing—review and editing. Zeid A. ALOthman: formal analysis, validation, visualization. Lee D. Wilson: writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassan, N.A.A.C., Hapiz, A., Jawad, A.H. et al. Desirability function and Box-Behnken design optimization for crystal violet dye adsorption by palm date stone activated carbon. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05710-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05710-2