Abstract
Agricultural waste is enriched with a variety of environmentally friendly materials that can potentially boost economic growth, reduce the spread of contagious diseases, and serve as a carbon-free renewable energy bioresource. Biopolymers produced from agricultural waste have a range of applications in medicine, agriculture, pharmaceutics, and industrial factories. The chemical extraction of biopolymers from biomass requires a series of alternating alkali, acid, and alkali treatments at controlled temperatures. Chemical extraction of plant-based biopolymers requires elevated temperatures (70–100°C), while for animal and sea organism-based biopolymers, moderate temperatures of 25–60°C are used. The obtained biopolymers are functionalized into various materials for application in a wide range of industries. The reported functional materials are loaded with inorganic nanomaterials, plant extracts, and organic compounds, which resulted in a synergistic effect and enhanced activity of the materials. Several researchers have synthesized biopolymers with synthetic polymers to improve their bioavailability, tensile strength, shelf life, and UV adsorption. This review article reports the extraction techniques of biopolymers from agricultural waste and their application in wound healing, water treatment, food storage, passive cooling, and cosmetics. The dearth of scientific articles on the applications of biopolymers generated from agricultural waste produced from food crops grown in Africa is a motivation for the present compilation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Agricultural production is a basic need in Africa, a continent of countries affected by severe poverty and lack of electricity and transportation. Most African countries heavily rely on agriculture for sustainable economic development and livelihoods, and the ever-growing population requires more agricultural production. Agriculture forms a large part of the economies of most African nations south of the Sahara; it contributes between 30 and 40% of the continent’s total economic output and provides a significant portion of the continent’s working population with a living. South Africa, Nigeria, Sudan, and Angola were reported to have the largest agricultural land area, equivalent to 292.4 million hectares, as of 2020 [1]. Maize is one of the staple foods in most African regions, and a record of 89.3 million metric tons was produced between 2020 and 2021, with the largest production coming from South Africa and Nigeria [2, 3]. About 25.4 million metric tons of wheat was produced in Africa in the years 2020–2021, with 60% of the production accounted for in Northern African countries [4]. The world population in 2018 was recorded at 7.3 billion and is expected to reach 8.545 billion by 2030, with the total energy consumption of 648 EJ [5]. Agricultural production in Africa is expected to increase by 11–30% between 2017 and 2029 due to its demand and population growth [6].
However, higher agricultural production leads to larger agricultural waste and residues (biomass) produced, including food wasted during the chain value. Although some of this agricultural waste is used as fertilizer and animal feed, 90% of it is distributed into landfills [7]. Seasonally, farmers dispose of or burn their waste after harvest, preparing the land for the next plantation [8]. Agricultural waste is often burned or dumped in fields, and this pollutes the air and the environment, thus attracting harmful microorganisms and insects that cause several health complications [9, 10]. The burning of biomass results in huge amounts of air pollutants (NOx, SO2, CO, and NH3) and greenhouse gases (CH4, CO2, and N2O) that contribute to global warming [11, 12]. In 2018, images captured by the Aqua satellite revealed fire burnings accounting for over a quarter of a million hectares across Africa [8]. Biomass is an essential raw material in renewable energy generation [13], biofuels [14], biopolymers, etc. This implies that the dumping and burning of biomass are a huge loss in promoting the economy and green environment. In Africa, there is limited research based on the extraction of biopolymers from agricultural waste and their potential use as functional materials.
Large macromolecules composed of a sequence of repeating units are known as biopolymers or natural polymers [15]. Owing to their sequential chain and functional groups, they can be used to covalently bond with active materials and deliver them to the targeted site. Biopolymers (cellulose, alginate, starch, xylan, carrageenan, chitosan, gelatin, hyaluronic acid, collagen, etc.) are polymers present in natural sources like plants or animals [16]. They are either derived from their source of origin through a series of chemical treatments or are naturally synthesized by living organisms [17]. Since they are derived from living organisms, they offer advantages such as being antimicrobial, biocompatible, biodegradable, environmentally friendly, non-toxic, bioadhesive, etc. [18, 19]. Owing to these characteristics, biopolymers can be transformed into functional materials like hydrogels, sponges, films, foams, patches, emulsions, lipids, and nanomaterials. The mentioned functional materials have a wide range of applications, such as food packaging, medical storage of pharmaceuticals, baby diapers, pollutant removal, house cooling, electric conductors, passive cooling, catalysis, agriculture, wound healing, and drug delivery[11, 20,21,22,23,24]. This review will focus on the extraction of biopolymers from biomass and their application as functional materials.
2 Biopolymers and sources
2.1 Agricultural wastes and their chemical composition
Agricultural wastes are leftovers generated after harvesting, processing of various agricultural products (plant- and animal-based), and spoilage resulting during packaging and the supply chain [5, 25]. They can be in the form of raw materials (leaves, seaweed, animal skins, stalks, shells, corn stover, etc.), by-products (bran, intestine, husk, bones, animal cutoffs, etc.), or a processed final product (bagasse) [26]. The biopolymers are found in different compositions based on their source of extraction (Table 1). Corn stover contains 11.7% lignin, 31.10% hemicellulose, and 40.67% cellulose [27]. Wheat straw consists of high levels of minerals (phosphorus and calcium), proteins, ash, silica, and carbohydrates (hemicellulose, cellulose, and lignin) [28]. Shrimp shells are the protective shield of shrimps and are composed of magnesium and calcium carbonate (20–50%), protein (20–40%), chitin (15–40%), and some minor constituents like minerals, lipids, and astaxanthin [29, 30]. The brown seaweed’s major constituents as dry weight include 3–15% of protein, 1–3% of lipids, 7–38% minerals, 50% carbohydrates (alginate, cellulose, and fucoidan), and 80–90% of water [31]. Bagasse, a waste product retrieved after the final processing of sugarcane, is rich in carbohydrates such as cellulose (32–48%), hemicellulose (25–32%), lignin (19–24%), raw extractive materials (5–9%), and ash (3%) [32,33,34].
2.2 Biopolymers and methods of extraction
2.2.1 Cellulose
Cellulose is an abundant biopolymer on earth, and it is present in the cell walls of most plants and some bacterial pathogens [35, 36]. Moreover, it is a source of renewable carbon from plants [37]. Owing to its abundance, non-toxicity, low cost, biodegradability, renewability, biocompatibility, good thermal stability, and mechanical strength, this biopolymer has attracted much attention for application in various fields [38, 39]. It is a linear polymer composed of a series of D-glucose molecules linked by β-(1→4) bonds (Scheme 1) [40]. Because of its strong hydrogen bonding, cellulose has poor solubility in most organic solvents [12, 35]. However, cellulose’s chain consists of hydroxyl functional groups, which can be easily modified to develop various cellulose derivatives that are soluble in aqueous solutions [33, 38]. The high content of hydroxyl groups present in cellulose allows it to be used as a superabsorbent material.
Cellulose is the most abundant polymer in plants such as cereal straws, wheat straws, corn stover, sugarcane bagasse, rice straws, etc. [41, 42]. Mzimela et al. reported the extraction of cellulose from sugarcane bagasse using five different methods involving the use of acidified and non-acidified sodium hypochlorite [43]. Mzimela and his group discovered that acidifying sodium hypochlorite using acetic acid to pH 4 yielded better crystallinity and thermal stability as compared to treatment with only sodium hypochlorite [43]. A similar method was reported by Senapitakkul et al.; however, they further treated the resultant powder with xylanase to improve its dyeability and remove excess lignin and hemicellulose [38]. Plermjai et al. obtained cellulose from bagasse by bleaching the bagasse using a 5% sulphuric acid solution (v/v), followed by treatment with 4% sodium hydroxide [41]. They further purified the obtained cellulose using acid hydrolysis assisted with milling and reported that this step improves the absorbability and crystallinity of the obtained cellulose [41].
Melesse et al. extracted cellulose from sugarcane bagasse using different concentrations of sodium hydroxide at controlled reaction times (15, 30, and 45 min) at 120°C [33]. After this step, 1% of the sodium chlorite solution was used to completely remove lignin at 95°C for 60 min. The sample was further treated with varying concentrations of sulphuric acid for 15 and 30 min under refluxing conditions at 100°C. The resultant product was then filtered and washed to a neutral pH using warm distilled water, followed by oven-drying overnight at 105°C. Gond et al. extracted 65% nanocellulose from sugarcane bagasse through a series of acid and alkali chemical treatments under controlled reaction conditions [44]. The obtained nanocellulose displayed antibacterial efficacy against E. coli and Bacillus bacterial strains, exhibiting 14.6- and 12.2-mm zones of inhibition, respectively. The characteristic features presented by films and composites made of the nanocellulose were suitable for application in plastic packaging, food packaging, and container fabrication.
Sugarcane bagasse is a common biomass used for the extraction of cellulose, and its cellulose content varies depending on the chemical treatment and reaction conditions. Notably, when bagasse is first treated with an 8–15% sodium hydroxide solution followed by a dilute strong acid solution (2–5% v/v), higher yields are obtained at around 80–100°C. Additionally, complete lignin removal is done through additional treatment with a 1–3% w/v sodium hydroxide solution between 80 and 100°C.
2.2.2 Chitosan
Chitin, the second most abundant biopolymer after cellulose, is derived from fungi, insects, and bacterial cell walls and has a higher composition in the exoskeletons of crustaceans [45]. The chemical extraction of chitin involves two steps, namely, demineralization (acidic treatment) and deproteinization (alkaline treatment) [30, 46]. Varun et al. obtained chitin from shrimp shells and deacetylated it to form chitosan, a major constituent of chitin [47]. They first ground and treated the grounded shells with hot water to remove dirt, followed by the 3-step extraction process. For demineralization, they treated the powder obtained from the pre-treatment step with 2N hydrochloric acid at room temperature for 2 h with constant stirring. The demineralized sample was then treated with a 2N sodium hydroxide solution agitated at 50°C for 2 h to remove proteins. The resultant chitin (14.72 ± 0.57% yield) was deacetylated using 50% sodium hydroxide with constant stirring at 121°C, 15 psi for an hour, followed by washing with distilled water to pH neutral to obtain chitosan (12.03 ± 0.46% yield). The obtained chitosan revealed FTIR characteristic peaks similar to those of commercial chitosan and displayed antibacterial efficacy against E. faecalis, S. aureus, E. aerogenes, and E. coli. This led to the confirmation of chitosan’s molecular weight to be <5000 kDA [47]. A similar chemical method was reported by Kandile et al. and Tokatlı et al. yielding similar chitin properties, and yields below 14% [48, 49].
Zhao et al. extracted chitin from shrimp shells using a different solvent method and compared it with the usual acid-alkali treatment method [50]. They used 10% citric acid in finely grounded shrimp shells to demineralize them, and then, they later treated the sample with different ratios of deep eutectic solvents (choline chloride:urea/glycerol/ethylene glycol, ratio 1:2) heated with microwave irradiation at different time intervals. The obtained chitin was compared to that obtained using the common acid/alkali treatment, and their treatment method was proven to be slightly significant, with a 25–22% yield compared to 17% yielded by the acid-alkali treatment. Both methods presented similar XRD, SEM, TGA, and FTIR results [50]. However, the use of 10% citric acid and deep eutectic solvents is an effective method to obtain higher yields of chitin and to limit the use of exotic acid and alkali solvents.
Xie et al. recovered chitin from shrimp shells using a two-step fermentation method involving bacterial pathogens [51]. Demineralization was achieved using L. acidophilus-containing fermentation medium shaken for 120 h in an incubator at room temperature. After complete demineralization, the fermentation medium was replaced with E. profundum fermentation medium, with conditions kept the same to remove proteins. The recovered sample was washed with deionized water and dried to obtain a 47.82% yield of chitin [51]. Tan et al. reported that the chemical composition of shrimp shells is about 30–60% minerals, 20–40% protein, and 20–30% chitin [52]. However, the bacterial fermentation method is an ideal procedure to recover higher yields of chitin, as reported by Xie et al. [51]. Chitin is structurally similar to cellulose and is composed of a series of linear β-(1→4) linked N-acetylglucosamine (N-acetyl-2-amino-2-deoxy-D-glucopyranose) units [41, 53]. Chitin, due to its high intermolecular hydrogen bonding, is highly insoluble in most organic and inorganic solvents and is often converted into chitosan [53, 54]. Chitosan is obtained by the deacetylation of chitin, and if the degree of deacetylation is higher than 50%, then it is termed chitosan [41]. Chitosan is a basic copolymer, and deacetylation of chitin results in the formation of amine groups, which give chitosan its cationic characteristic [18, 53, 55]. Chitosan is composed of D-glucosamine and N-acetyl-β-D-glucosamine units linked by β-(1→4) bonds [56, 57]. It is insoluble in water; however, it is soluble in most acidic aqueous solutions [36, 54].
2.2.3 Alginate
Alginate or alginic acid is an insoluble natural biopolymer recovered from the cell walls of seaweed but is in higher composition in the cell walls of brown seaweed [58]. However, various methods have been employed to extract a water-soluble form of alginate (sodium alginate). Alginate is composed of a series of α-L-guluronate (G-blocks) and β-D-mannuronate (M-blocks) linked by 1→4 glycosidic bonds [59, 60]. The sequence and arrangement of G- and M-blocks are determined by their source of extraction [61]. The G-blocks of alginate interact with calcium ions to form an egg-box-like structure hydrogel [58, 61, 62]. Smith et al. reported the gelling and swelling properties of alginate-based materials to be influenced by a higher number of M-blocks over G-blocks [18]. The chemical structure of alginate contains hydroxyl and carboxyl groups, which are the targeted sites of reaction and modification [63]. It has a high gelling ability, allowing it to be functionalized into various value-added materials like gels, beads, sponges, microspheres, films, hydrogels, etc. [58, 59]. The common chemical extraction of sodium alginate includes the removal of fucoidan through acid treatment [64], the conversion of alginic acid into sodium alginate through alkaline treatment, solid/liquid separation, and precipitation [65].
Trica et al. extracted sodium alginate using the acid/alkali treatment method, in which Cystoseira barbata (a specie of brown seaweed) was used [66]. They treated the dried seaweed powder with 70% ethanol for 24 h at room temperature to remove fat and pigment. The depigmented powder was then treated with 0.1M hydrochloric acid for 2 h at 60°C to remove fucoidan, and this step was repeated twice. The obtained powder, which is the insoluble alginic acid, was treated with a 3% sodium carbonate solution for 2 h at 60°C to yield a water-soluble sodium alginate. This solution was centrifuged to separate the contaminants from the sodium alginate solution. This alginate-containing solution was precipitated with 96% ethanol at −18°C to recover a yield of 19±1.5% of dry sodium alginate. Jayasinghe et al. used Sargassum wightii brown seaweed to obtain sodium alginate [67]. The dried Sargassum wightii meshed samples were dipped in a 1% calcium chloride solution, followed by dipping in 1% formaldehyde to achieve depigmentation for 2 and 6 h, respectively. The sample was then washed with deionized water and dipped in 5% hydrochloric acid for 30 min to obtain the alginic acid. This alginic acid was then treated with a 2.5% sodium carbonate solution for 3 h and filtered to remove the solid from the liquid phase. This solution was then precipitated with 95% ethanol under constant stirring, and the recovered precipitate was dried in an oven to obtain ±31.5% yield.
Nat et al. obtained ±17% sodium alginate from Sargassum angustifolium brown seaweed [68]. The S. angustifolium powder was defatted and depigmented using a solution mixture of methanol, chloroform, and water at room temperature overnight, followed by treatment with 80% ethanol. This was then treated with a 0.1 M hydrochloric acid solution to remove fucoidan at 60°C for 3 h and this stage was repeated twice. A solution of 2% sodium carbonate was used to treat the residual obtained after acid treatment for 4 h at 60°C. The resultant solution was filtered to remove any solid materials and precipitated with absolute ethanol to obtain the desired sodium alginate. The obtained sodium alginate was purified using deionized water and an absolute ethanol solution and washed with acetone before drying.
Dalal et al. derived sodium alginate from algal biomass [69]. The algal biomass was dried and crushed, and then, 20g of it was washed in boiling water (300 mL) for half an hour. This was then boiled in a 0.5% calcium chloride solution to stabilize the alginate/alginic salt in the algal biomass. The resultant was then boiled for 1 h in 0.5% sodium chloride. The obtained filtrate, after boiling, was boiled again for 30 min in 5% sodium carbonate with continuous stirring. After this step, the obtained product was finally filtered and precipitated with 80% ethyl alcohol, followed by drying and storage.
2.2.4 Gelatin
Gelatin is a naturally occurring biopolymer derived from either alkali or acid hydrolysis of collagen from pigs, cows, fish scales, etc. [45]. Acid treatment of collagen results in type A gelatin, and alkali treatment yields type B gelatin [70]. Type B gelatin has a slower degradation rate compared to type A, which could be linked to its higher cross-linking degree [71]. Gelatin has a large polypeptide structure [72], consisting of 18 amino acids, of which proline, hydroxyproline, and glycine constitute ±57% and other amino acids ±43% [36, 71]. Gelatin forms gels below 30–40°C and the dominance of the mentioned amino acids in its structure promotes its bio-adhesion properties [36, 70, 73]. Moreover, the high amino acid structure aids in gelatin’s physical and biological properties, such as being biocompatible, being biodegradable, low toxicity, ease of functionalization, etc. [36, 72]. Due to these advantages, gelatin has been widely used to prepare functional materials in various fields, mostly in food and tissue engineering.
Saenmuang et al. extracted gelatin from black-bone chicken skin and feet and recovered 7.23 and 6.60%, respectively [74]. The chicken by-products were first defatted, cleaned, chopped into small pieces, and freeze-dried. They then used different concentrations (0.025, 0.050, 0.075 N) of 200 mL sodium hydroxide solution for pre-treatment of collagen; the reaction proceeded for 80 min under continuous slow stirring at 22°C. They treated the product obtained with a 0.15% sulphuric acid solution followed by 0.7% citric acid for 40 min with constant stirring, repeated three times. After every step, the samples were centrifuged for 10 min and rinsed with deionized water. In the final step, the samples were soaked in distilled water at 45°C for 15 h followed by cooling and mixing with activated carbon for 20 min. The mixture was filtered, and the filtrate was concentrated and freeze-dried to obtain powdered gelatin. In their study, they discovered that the yield of gelatin is influenced by the concentration of sodium in the pre-treatment step.
Similar studies were reported by Chakka et al., who derived gelatin from chicken feet [75]. In their report, the chicken feet were minced into a paste with a meat mincer before pre-treatment with 0.5 M sodium hydroxide for 20 h. This was then filtered, and the residual was washed to pH neutral with water, followed by treatment with different concentrations (1.5, 3, and 4.5%) of lactic acid, acetic acid, and citric acid overnight. These solutions were then heated for 20 min at 55°C, then filtered, and the filtrate was freeze-dried to obtain a gelatin powder. The concentration of the acids significantly impacted the percentage yield, as the yield of gelatin increased with an increase in acid concentration. In summary, the lactic acid treatment exhibited the highest yield of 14.47% compared to other forms of acid treatment. An increase in the acid concentration of lactic and acetic acid negatively influenced the content of hydroxyproline, while citric acid revealed a directly proportional relationship [75]. Higher hydroxyproline composition and gel strength were recovered using 1.5% acetic acid. The overall chemical composition of amino acids present in gelatin at 1.5% acetic acid displayed high glycine content, followed by proline, alanine, hydroxyproline, and other amino acids.
Rather and co-workers [76] used and modified the method reported above by Chakka et al. [75] to extract gelatin from chicken feet. In their study, the pre-treatment step was achieved in 2 h using similar conditions, and they defatted the sample with 10% butyl alcohol. After the removal of fat, the sample was then treated with a 0.1 N hydrochloric acid solution with constant stirring for 24 h. After this step, the sample was soaked in a 4.5% lactic acid solution overnight. The solution was then heated at 55°C followed by filtration, and the filtrate was hot air-dried at 45°C or freeze-dried at −60°C. The obtained percentage yield was not significantly different from that reported by Chakka et al. and was 14.5 and 15.7% for hot air-dried and freeze-dried gelatin, respectively. The gelatin produced in this study showed reasonable gelling, stabilizing, foaming, emulsion capacity, and oil-binding capacity properties. Their findings were not significantly different from the findings reported by Chakka and co-workers, suggesting that the average composition of gelatin from chicken is ~14.8% using the above-mentioned method. Kamatchi et al. used fish skins of Sphyraena barracuda and Carcharhinus amblyrhynchos, and recovered 6 and 8% yields of gelatin, respectively [77]. The skins were immersed for 40 min in 0.2% sodium hydroxide, followed by step-by-step treatment with 0.2% sulphuric acid and 1% citric acid. This was then washed with cold water and soaked in distilled water for 18 h at 45°C. The resultant solution was filtered, and the filtrate was evaporated at 70°C followed by hot air oven-drying at 50°C. The yield of gelatin from its source is affected by the concentration of the organic solvents used during pre-treatment steps and the temperature.
Amertaning and co-workers derived gelatin from cattle skin using both acid and alkali treatments [78]. They scraped the fur off the animal skins and chopped them into medium pieces before freeze-thawing them for 6 h and further cutting them into smaller pieces. The small samples were soaked in separate acid 0.25 M and alkali 0.25 M solutions for 3 days, followed by washing and soaking in distilled water for 6 h at 55°C to extract gelatin. This solution was filtered, and the filtrate was dried to obtain 11.04% gelatin recovered from acid treatment and 5.42% from basic treatment. The acid treatment produced more efficient results compared to the alkali treatment. Nevertheless, alkali treatment revealed a high yield of amino acids with a high composition of glycine, followed by glutamic acid and arginine [78].
Asif et al. obtained gelatin from camel hides by first treating the hides with sodium hydroxide. A sodium sulphide solution was used to remove hair at 10°C for 3 days, followed by cutting it into pieces [79]. The hides were then soaked in 0.5 M sulphuric acid for another 3 days, followed by washing with distilled water and pH adjustment (pH 5.26). This was then soaked for 3–5 h in distilled water at 71.87°C. The solution was then filtered, purified, and dried to obtain a 29.1% yield of gelatin.
3 Applications of biopolymer-based materials
The aforementioned properties of biopolymers enable them to be functionalized into various functional materials for application in different fields of research, such as tissue and wound healing, water treatment, recovery of metals in mines, food storage, drug delivery, cooling systems, sanitary products, and cosmetics, among others [16, 80,81,82].
3.1 Biopolymers in cosmetics
Mondejar-Lopez et al. developed chitosan-based nanoparticles encapsulated with varying concentrations (0.25, 0.5, 0.75, and 1) of carvacrol or eugenol as preservatives in cosmetics [83]. The encapsulation efficiency of these nanoparticles ranged between 33.4 and 3.7% with loading efficiency between 25 and 2.4% and particle size between 126 and 50.2 nm. Carvacrol-encapsulated chitosan nanoparticles (CH-Carv) displayed a zeta potential of +26mV which was higher than that of eugenol-containing nanoparticles (CH-Eug) −25mV, signifying that CH-Carv nanoparticles are more stable than CH-Eug nanoparticles. The stability of these formulations decreased with an increase in the concentration of the encapsulated materials.
Drug release profiles showed a biphasic and fast release mechanism for CH-Eug, while a relatively triphasic profile was observed for CH-Carv with slow carvacrol release. The drug release profiles played a significant role in the inhibition of microorganisms (A. brasiliensis, C. albicans, E. coli, P. aeruginosa, and S. aureus) associated with cosmetic product invasion. CH-Eug displayed the highest antimicrobial activity against the tested pathogens, showing a minimum inhibition concentration (MIC) of 52 μg/mL against P. aeruginosa, while CH-Carv exhibited a MIC of 222 μg/mL against S. aureus. Encapsulation of the preservative agents significantly enhanced their antimicrobial activity, as lower MIC values were observed for CH-Eug and CH-Carv compared to their free forms. However, CH-Carv displayed no significant difference from free carvacrol as they exhibited similar MIC values against the fungal pathogens (A. brasiliensis and C. albicans). Moreover, the antioxidant results displayed 67.7% antioxidant activity for CH-Eug compared to 45.9% for CH-Carv.
These nanoparticles were then formulated into moisturizing cream to evaluate their preservative activity with a blank cream used as a control. The control cream did not present any preservative activity against all the treated pathogens, as an increase in the colony-forming units (CFU) of the pathogens was observed. The addition of CH-Carv to the cream decreased its antimicrobial activity, as it did not show any activity across the treated pathogens. However, 7 days post-treatment, CH-Carv cream exhibited activity against A. brasiliensis. CH-Eug displayed a total reduction against A. brasiliensis, E. coli, and C. albicans, while P. aeruginosa and S. aureus displayed resistance, though a slight reduction of 1 log in CFU was displayed. These results suggest that the fast release displayed by CH-Eug aided in its antioxidant, microbial, and preservative activity, signifying that it is an ideal material for the preservation of cosmetic products. However, additional studies should be employed to further confirm the safety of the materials for application to the skin.
Cho et al. used the biomass of Hesperethusa crenulata heartwood to extract cellulose [84]. The obtained cellulose was used to develop a hydrogel film-facial mask loaded with Thanaka extract. The facial mask was prepared by soaking the cotton mask in the cellulose solution, and this was later stored in a bag filled with Thanaka extract. The water retention content of the plain cotton was 73.1%, and that of the hydrogel film was 165.5%. However, this was significantly increased to 465.6% for the facial mask, showing high water retention ability, which is a good feature for facial treatment products. They likened their hydrogel film mask to resembling human skin tissue due to its high flexibility, mechanical properties, and water retention content. The antimicrobial activity of the hydrogel film was tested against B. pumilus, B. subtilis, S. aureus, P. aeruginosa, E. coli, and C. albicans using the disc agar-well diffusion method. The hydrogel film mask exhibited an average diameter of 18 mm against B. subtilis, S. aureus, and E. coli, followed by 17 mm for B. pumilus, 15 mm for P. aeruginosa, and 12 mm for C. albicans. The obtained facial mask was then evaluated for its facial treatment potential on a female human being. The mask was put on the face and taken off after 20 min; then, the face was rinsed with water, and smooth and bright skin was observed after application for 2–3 times a week.
Gaspar et al. developed chitosan bioactive films incorporated with lemongrass essential oil (LEO) for potential skincare applications [85]. Various concentrations of LEO were incorporated into the films, and it was noted that by increasing the concentration of LEO, a more yellowish film was observed. Moreover, the water vapour permeation rate decreased as the concentration of Leo increased, which could be linked to the hydrophobic nature of oils, which is an interesting feature for cosmetic masks used for the treatment of wrinkled skin. The prepared films displayed homogeneity and flexibility and they maintained their texture after 1 h of soaking in distilled water without dissolving, a time that mimics that of the mask’s application on the skin before removal. The antioxidant activity of the films was similar to that of N-acetyl-L-cysteine, a prodrug widely used as an antioxidant. Additionally, a HaCaT cell viability of 70% was observed in cells treated with film loaded with 0.5% of LEO. Higher concentrations LEO significantly decreased the viability of HaCaT cells. These scaffolds further inhibited the bacterial growth of E. coli and S. aureus, showing that they can be used as ideal masks for skincare and antiaging.
Ta and co-workers developed chitosan nanoparticles cross-linked with acacia or sodium tripolyphosphate (TTP). The cross-linkers were used to enhance the delivery and penetration of cosmetic constituents through the skin [86]. The reported nanoparticles had an average size of 200–300 nm and they showed an average of 98% water retention. The particle size of the nanoparticles was dependent on the concentration of both chitosan and cross-linkers used. These nanoparticles further displayed lower moisture retention while showing high moisture absorption, an important parameter in maintaining the skin’s moisture and keeping the stratum corneum hydrated. This implies that as the nanoparticles absorb moisture from their environment, the polar active ingredients will be entrapped within the nanoparticle’s network and released into lower-polarity skin conditions. The nanoparticles were non-toxic to human dermal fibroblast cells, exhibiting a minimum of 80% cell viability at the highest concentration tested. Porcine pig ear skin was used to determine the cellular uptake, and it was discovered that the nanoparticles were able to penetrate and gather in the dermis layer. The findings presented in this study suggest that chitosan nanoparticles are good functional materials for the delivery of cosmetic active ingredients through the skin while maintaining the skin’s moisture.
The potential activity of the cosmetic materials reported has been tested in vitro and on humans. The reported materials showed efficacy and safety both in vitro and on human skin.
3.2 Biopolymers in passive cooling and storage
Climate change has led to increased temperatures leading to quick decomposition of food, increased usage of water, drought, decreased survival rate of livestock, etc. The development of passive cooling systems to limit the effect of elevated temperatures is needed. Feng et al. developed acrylamide-based bilayer hydrogels for the passive cooling of buildings [87]. The bilayer porous film was composed of a hydrophobic top layer and an inner hydroscopic hydrogel layer. The top layer was designed to protect the hydrogel layer from direct UV light, thus enhancing its cooling effects during the day while allowing it to recover during the night. The reported bilayer displayed an ambient cooling power of ~150 W·m−2 and approximately 7°C of temperature drop under direct sunlight. This is a promising approach to the development of cost-effective cooling systems and the reduction of electricity consumption.
Ferber et al. developed hydrogel-based storage materials composed of poly(N-isopropylacrylamide) (PNIPAM) for cooling biologics [88]. The functional properties of the PNIPAM hydrogels were compared to those of polyacrylamide (PAM) hydrogels. When PNIPAM and PAM hydrogels were incubated at 36°C with 50% humidity for 7 days, PAM hydrogels displayed an effective cooling performance over the first 3 days compared to PNIPAM hydrogels. However, proceeding to day 7, the water content of PAM hydrogels significantly dropped, while PNIPAM hydrogel retained its water content and maintained a lower stable temperature. The PAM hydrogels dried out and broke by day 3, while PNIPAM managed to retain their water. The PNIPAM hydrogels exhibited full recovery when rehydrated.
The hydrogels were further incubated at 39 and 12°C to mimic the day and night temperature conditions. PAM hydrogels failed to withstand the alternative change of temperature after two cycles due to a loss of water content. However, PNIPAM hydrogels maintained a lower core temperature for up to 5 days. Three serotypes of the oral polio vaccine (OPV) were used to determine the evaporative cooling potential of the hydrogels, with serotype 1 being the most temperature-sensitive, serotype 2 being the most thermostable, and serotype 3. Under stable conditions (36°C and 50% humidity in 7 days), PNIPAM hydrogels displayed higher preservation and recovery of OPV across the tested serotypes compared to unpackaged and PAM hydrogel-treated serotypes. However, at serotype 1, there was no statistical difference between the treatment groups. Moreover, PNIPAM hydrogels exhibited significant recovery of OPV serotypes under cycling day and night conditions in 7 days, compared to other treatment groups. The reported hydrogels are promising scaffolds for the storage of thermolabile pharmaceuticals without a refrigerator.
Lu et al. developed a hydrogel-aerogel bilayer material for application in passive cooling [89]. The aerogel layer was composed of hydrophobic silica, and the hydrogel layer was composed of 2-acrylamido-2-methylpropan sulfonic acid and acrylamide. The reported bilayer prolonged the lifetime of the cooling package by 400%, as matched with the single-layer method design. These bilayer materials were suitable for the preservation of food, the storage of pharmaceuticals, and the cooling of buildings. Riaz et al. developed alginate-containing double network (DN) hydrogels and evaluated their cooling potential against brick and wooden houses [90]. When investigated under direct sunlight at 47°C, the experimental brick house displayed a temperature of 31°C, while the wooden house maintained a temperature of 39.5°C. These results show that these functional materials can promote evaporation and cooling effects without the consumption of electricity.
Lv and co-workers developed bio-inspired hydrogels and evaluated their cooling efficacy on photovoltaic (PV) panels under normal working conditions [91]. They reported that using 0.5 g of the hydrogel, a cooling power of 70 Wm−2 was achieved for a standard 6-inch PV panel. This was achieved without weakening the conversion efficacy. The hydrogels further displayed a 5°C temperature drop for the panels under normal working conditions. Xu et al. developed nanocomposite hydrogel-based daytime passive cooling systems for application in food preservation and building cooling [92]. They reported that nanocomposites with approximately 0.92 atmospheric emissivities and 0.95 solar reflectances could realize daytime radiation. The reported nanocomposite hydrogels exhibited excellent flexibility, mechanical strength, rehydration, and swelling ability. The adsorption properties of these hydrogels significantly promote radiative and evaporative cooling. Under direct sunlight at 35°C, the hydrogels exhibited temperature drops in the range of 2.9–6.2°C.
3.3 Biopolymers in metal absorption
Clean water is a basic need of life, and accessible water in most river dams is often polluted with dyes, surfactants, plastics, oils, heavy metals, etc. This often poses a danger to human lives, as some still use contaminated water for drinking, livestock, and gardening. Polluted water is a serious issue affecting most African countries, and an estimation of 115 people die per hour as a result of diseases linked to contaminated water, poor sanitation, and improper hygiene [93]. Water treatment is a necessity to improve human health, and the use of polymer-based materials in water treatment has attracted a lot of researchers. Biopolymers in water treatment offer the following advantages: they are cheap to synthesize, have high absorption properties, can be functionalized into different sizes with varied chemistry, and are safe. Sharmila and co-workers reported sodium alginate beads for the treatment of textile wastewater [94]. They discovered that the absorption efficiencies of the reported beads were influenced by the pH, flow rate, and diameter of the beads. The optimum removal of nitrates was 75%, 56% for alkalinity, and 32% for sulphates at a flow rate of 50cc/hr, pH 9, and a bed height of 10 cm. Bustos-Terrones et al. reported sodium alginate-based beads exhibiting 99.5% degradation of basic blue 9 dye in textile wastewater after 2 h of treatment [95]. These functional materials also showed 71% and 93% degradation of organic matter and phosphorous in domestic wastewater after 12 h.
Trica and co-workers, in their study, developed alginate-based beads for heavy metal absorption in water [66]. The average mean diameter of the reported beads was recorded at 4413 ± 134 μm, and the absorption properties of these beads were compared to those of the alginate source biomass. They discovered that the biomass managed to absorb heavy metals (lead and copper) from the water, exhibiting metal absorption capacities of 69.3 ± 2 mg/g and 279.2 ± 7.5 mg/g for Cu2+ and Pb2+, respectively. Functionalization of alginate into beads greatly improved its metal absorption potential, showing a maximum of 107.3 ± 1.7 mg/g for Cu2+ and 454 ± 4.7 mg/g for Pb2+ as estimated by the Langmuir absorption model. The absorption mechanism of these beads was linked to the ion exchange of Ca2+ in the alginate network, which was replaced by Cu2+ or Pb2+ as they have higher affinity. Findings reported in this study suggest that the produced beads have the potential to be applied in the treatment of water contaminated with heavy metals.
Zhang et al. reported hydrogel beads loaded with polyaniline-polypyrrole modified graphene oxide for the removal of Cu2+ and Cr(VI) from water [96] (GO-PAN-PPy). The incorporation of GO-PAN-PPy led to a larger pore size and improved absorption kinetics of the hydrogels. The absorption kinetics were also influenced by the pH, as maximum removal of Cr(VI) was observed at pH 2 and pH 6 for Cu2+. The absorption capacity of these hydrogels was affected by various factors; as observed, the increase in concentration of GO-PAN-PPy negatively influenced the absorption of Cu2+ and promoted that of Cr(VI). The absorption potential for Cr(VI) and Cu2+ was estimated at ~133.7 mg/g and ~87.2 mg/g, respectively, at pH 3. The absorption mechanism of Cu2+ was achieved through ion exchange and complexation, while Cr(VI) was sequestered via reduction, ion exchange, hydrogen bonding, electrostatic interactions, and complexation of the adsorbate. The findings showed that the resultant absorbent materials can be applied to the removal of ion-charged metals from water.
Kim et al. extracted cellulose from paper mulberry through a soda pulp process and functionalized it into a flocculant to treat wastewater polluted by surfactants from cosmetic products [97]. They targeted linear alkylbenzene sulfonates (LASs), the commonly used cosmetic anionic surfactant in showers and bath products [97]. The LAS solution was treated with different concentrations (1%, 2%, and 3%) of the coagulant (FeCl3·6H2O) and the removal efficacy was directly proportional to an increase in concentration from 16.04 to 94.01%. The removal efficiency displayed by 1% coagulant was significantly improved to 92.73% when 0.3% cellulose-based flocculant was added to the solution. It was also noted under these exact conditions that pH adjustment is also a critical parameter to be considered for optimum removal of LAS, as a pH of 2 exhibited 95.62% removal efficiency. The lowest recorded LAS removal was at pH 7 (88.68%); however, it was not statistically different from that at pH 10. The mechanism of LAS removal in water is linked to the high number of carboxyl groups in the flocculant. This study displayed an eco-friendly and cost-effective approach for wastewater treatment using lower concentrations of the flocculant and coagulant.
Bilici et al. treated wastewater polluted with waste motor oil with calcium alginate-based beads loaded with different concentrations (25, 50, and 100 mg/L) of sodium dodecyl sulphate (SDS) [98]. The binding efficiencies of SDS to the beads decrease with an increase in their concentration, revealing 84% at 25 mg/L and 48% at 100 mg/L. In a range of pH 2–10, the effective pH for maximum oil absorption was recorded at pH 6–8, exhibiting 77% absorption efficiency. The absorption capacity of the beads increased significantly from 77 to 95% when the concentration of beads was increased from 2.5 to 30 g/L. Additionally, under the same conditions, it was noted that smaller particle sizes (<1 mm) of the beads increased oil absorption, while larger sizes of 4 mm displayed lower absorption efficiency. The absorption kinetics of the beads displayed pseudo-second-order kinetics, as an increase in oil concentration from 25 to 1000 mg/L led to 8.34 to 22.12 mg/g oil molecule absorption. The beads were best fitted to the Langmuir isotherm model. These beads were reusable, and their absorption capability only decreased by 5% after 7 reuses.
Several researchers have developed and reported efficient water treatment approaches. However, there is limited information on the commercialization of these materials and their possible application on a larger scale.
3.4 Biopolymers in wound healing
Biopolymers in wound healing are mostly used as delivery systems of active materials into the affected site. Nevertheless, some biopolymers possess natural wound healing characteristics such as being antibacterial, adhesive, and analgesic, promoting wound healing factors, less scaring, etc. Wound healing is an important and life-saving process after injury, as the body often leaks blood leading to various complications. Wounds are prone to infection, and without proper treatment, they may expand, compromising the immune system and sometimes leading to amputation. Biopolymers are easily recognized by the body, and due to their properties mentioned above, they are suitable functional materials to overcome limitations associated with wound healing and thus promote rapid wound closure. Buyana et al. developed topical gels using sodium alginate and pluronic F127 and loaded them with bioactive materials (aminocaproic acid, norfloxacin, thymol, and zinc oxide) [99]. In their study, they discovered that loading both zinc oxide (ZnO) and aminocaproic acid into the gels led to improved blood clotting kinetics of the gels, showing a synergistic effect between the loaded active materials and the polymer network. The reported gels also showed a broad spectrum of antibacterial activity against both gram-negative and gram-positive pathogens.
Mehrabi et al. developed borate bioactive glass (BBG)-loaded chitosan-carboxymethylcellulose-based hydrogels for the management of wounds [100]. The reported hydrogels displayed a fast degradation rate in the first 7 days, which was then sustained from days 7 to 14 at 37°C, signifying they can be applied to the body for a prolonged period time. The antibacterial activity of the hydrogels was due to the loaded BBG, as plain hydrogels displayed no sign of bacterial inhibition. The MTT assay displayed that the hydrogels promoted the cell viability of mesenchymal stem cells prior to 7 days of treatment. Moreover, the reported gels significantly promoted wound healing in vivo, showing 79.27% wound reduction in male NMRI mice after 7 days post-injury and 96.7% after 14 days.
Shah and co-workers reported diabetic wound healing effects of carboxymethylcellulose/chitosan-based hydrogels impregnated with nano-curcumin [101]. The percentage entrapment efficiency and drug loading content were above 95% for all the reported formulations in this study. The hydrogels exhibited a cell viability of 80% for 3T3-L1 cells. The wound healing potential of the hydrogels was first evaluated using a wound scratch assay, and complete cell migration of 3T3-L1 cells was observed after 72 h of treatment with the reported hydrogels. Using male Wistar rats, complete wound healing was observed after 20 days when treated with the functional hydrogels. The healed area appeared to have hair follicles, sebaceous glands, sweat glands, and blood vessels.
The activity of the reported scaffolds was tested in vitro and in vivo and they revealed promising wound healing effects. However, preclinical studies are needed to confirm safety of these materials.
3.5 Biopolymers in the food industry
Food storage and preservation are one of the limitations associated with the food and market industries. Food after harvesting is accompanied by various factors such as bacterial invasion, fungal infection, temperature, handling and storage, processing, etc. [26], leading to its spoilage. Various methods have been practised to overcome factors associated with food spoilage, including canned food, refrigerators, plastic containers, and more. However, these methods are often associated with environmental protection, food safety, poor biodegradability, energy, etc. [20, 102]. The use of biopolymers in food preservation and storage is a promising approach, as they contain and deliver bioactive materials, inhibit microbial infection, allow gaseous exchange, provide cool temperatures, are non-toxic, promote food safety, etc. [103].
Zhao et al. prepared functional composites composed of silver nanoparticles (Ag-NPs) and grape seed extract (GSE) and coated them with chitosan as functional materials for food packaging [104]. These functional composites had an average particle size of 10–30 nm with a spherical shape. The antioxidant and antimicrobial activity of the composites was influenced by their concentration in the treatment medium. A high concentration of 100 μg/m displayed maximum antioxidant scavenging activity and microbial inhibition of ±75% and ±17.0 mm diameter zones of inhibition of P. chrysogenum and A. niger fungal pathogens, respectively. After 5 days of treatment with the reported composites at 20°C, the harvested grapes maintained their quality, while other treatment groups displayed decay, dehydration, and fungal infection. The good preservative properties displayed by these composites were due to the synergistic effect between Ag-NPs, GSE, and chitosan. These composite materials further displayed reduced weight loss and rotting percentage, inhibited total mould count, and maintained the titratable acidity of the stored grapes. The functional materials produced in this study have the potential to be used as packaging materials for the elongated shelf life and storage of fruits.
Zhai et al. developed nanocomposite films composed of starch and poly(butylene adipate-co-terephthalate), incorporating a combination of ZnO-NPs and Ag-NPs [105]. Composite films containing both ZnO and Ag-NPs exhibited higher points at elongation and tensile strength than films loaded with only a single nanoparticle. Films containing both nanoparticles also showed a synergistic effect against microbial inhibition of S. aureus and E. coli with over 95% efficacy within 3 h. The preliminary packaging studies were compared to those of commercial low-density polyethylene (LDPE) films. The freshly produced nectarines and peaches displayed no signs of spoilage after 9–4 days of storage treatment with films containing 0.8% Ag-NPs and 0.2% ZnO-NPs at 23°C and 53% relative humidity compared to LDPE, with visible spoilage, dehydration, and shrinkage. Moreover, the reported films prevented the penetration of UV light. These functional materials are suitable for the preservation and packaging of food, as they increase shelf life and maintain the quality of foods.
Liu and co-workers developed chitosan and gelatin-based (CGP) hydrogel films loaded with 3-phenyllactic acid as food packaging materials to extend the shelf life of chilled chicken [106]. The antibacterial activity of the reported CGP hydrogels increased with an increase in the concentration of 3-phenyllactic acid (3-PLA) against S. aureus and E. coli. These hydrogels exhibited >90% cell viability in Caco-2 and SW480 cells, and the chilled chicken breast was used to evaluate the potential storage time of CGP films. The reported films adhered and absorbed water on the surface of the chicken breasts after wrapping, forming a protective membrane. After 6, 8, and 10 days, the pH of the chicken breasts was significantly increased for the control and films without 3-PLA, whereas CGP-1-treated breasts displayed minimal pH changes. Using total volatile basic nitrogen (TVB-N) as a spoilage indicator, the control and plain film groups presented TVB-N values above 25 mg/100 g after 8 days, while CGP-1 displayed values below 20 mg/100 g, showing second-level freshness. The bacterial count of chicken breasts treated with control and plain films reached 6 log CFU/g, while for CGP-1-treated chicken breasts, it was 4.23 log CFU/g after 4 days of treatment. The total bacterial counts were directly proportional to the increase in time.
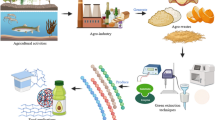
The reported materials have potential for application in the storage and preservation of food. However, continual studies are needed for the application of these materials on a broader market scale. Fig. 1 displays a schematic summary of the work reported in this review.
The agro-waste and its application as biopolymer-based functional materials in industries [107]
4 Conclusion and future perspectives
The extraction of biopolymers from biomass is one of the vital approaches contributing to safe and green chemistry due to their wide application in various industries. The extraction of biopolymers often requires various chemical treatments with diluted acid and alkali solutions at controlled temperatures. Contrary to other polymers, the extraction of cellulose has elevated reaction conditions (80–100°C) and a stronger alkali solution, resulting in complete degradation and removal of lignin. Among the various chemical treatments, the bacterial treatment method is ideally the most effective in order to recover higher yields of the biopolymers. These biopolymers can be functionalized into various functional materials for application in broad day-to-day life. The activity of biopolymer-based materials is influenced by loading them with nanomaterials, plant extracts, and organic and inorganic materials. Loading biopolymer-based materials with bioactive compounds resulted in a synergistic effect and improved effectiveness. Their efficacy was proven in vitro and in vivo, and it can be concluded that their biocompatibility, swelling ability, antibacterial activity, and absorbability play an all-round role across the reported industrial applications. However, further studies are needed for the potential application of these materials for day-to-day purposes and their market availability.
Data availability
The data is cited and included in this manuscript.
References
Africa: agricultural land area by country | Statista (n.d.) https://www.statista.com/statistics/1305411/agricultural-land-area-in-africa-by-country/. Accessed 4 May 2023
Africa: production volume of corn 2017-2022 | Statista (n.d.) https://www.statista.com/statistics/1294303/production-volume-of-corn-in-africa/. Accessed 4 May 2023
Africa Maize Market Size & Share Analysis - Industry Research Report - Growth Trends (n.d.) https://www.mordorintelligence.com/industry-reports/african-maize-market. Accessed 4 May 2023
Africa: production volume of wheat 2017-2022 | Statista (n.d.) https://www.statista.com/statistics/1294190/production-volume-of-wheat-in-africa/. Accessed 4 May 2023
Awogbemi O, Von KDV (2022) Valorization of agricultural wastes for biofuel applications. Heliyon 8:e11117. https://doi.org/10.1016/j.heliyon.2022.e11117
Agriculture in Africa 2021 (n.d) https://catalogue.unccd.int/1701_OCP_Agriculture_Africa_Report_2021.pdf. Accessed 4 May 2023
Turning Africa’s food waste problem into a regenerative opportunity - Climate Champions (n.d.) https://climatechampions.unfccc.int/turning-africas-food-waste-problem-into-a-regenerative-opportunity/. Accessed 4 May 2023
Agricultural Fires Seem to Engulf Central Africa | NASA (n.d.) https://www.nasa.gov/image-feature/goddard/2018/agricultural-fires-seem-to-engulf-central-africa. Accessed 4 May 2023
Seroka NS, Taziwa R, Khotseng L (2022) Green synthesis of crystalline silica from sugarcane bagasse ash: physico-chemical properties. Nanomaterials 12:1–13. https://doi.org/10.3390/nano12132184
Mokhena T, Mochane M, Tshwafo M, Linganiso L, Thekisoe O, Songca S (2016) agricultural solid wastes: causes, effects, and effective management. intechopen:225–240
Li S, Chen G (2020) Agricultural waste-derived superabsorbent hydrogels: Preparation, performance, and socioeconomic impacts. J Clean Prod 251:119669. https://doi.org/10.1016/j.jclepro.2019.119669
Miljković V, Gajić I, Nikolić L (2021) Waste materials as a resource for production of cmc superabsorbent hydrogel for sustainable agriculture. Polymers (Basel) 13. https://doi.org/10.3390/polym13234115
Potgieter JG (2011) Agricultural residue as a renewable energy resource utilisation of agricultural residue in the greater gariep agricultural area as a renewable energy resource. Dissertation. Stellenbosch University
Stafford WHL, Lotter GA, von Maltitz GP, Brent AC (2019) Biofuels technology development in Southern Africa. Dev South Afr 36:155–174. https://doi.org/10.1080/0376835X.2018.1481732
Baranwal J, Barse B, Fais A, Delogu GL, Kumar A (2022) Biopolymer: a sustainable material for food and medical applications. Polymers (Basel) 14:1–22. https://doi.org/10.3390/polym14050983
Christian SJ (2016) Natural fibre-reinforced noncementitious composites (biocomposites). In: Harries KA, Sharma B (eds) Nonconventional and vernacular construction materials. Elsevier, pp 111–126. https://doi.org/10.1016/b978-0-08-100038-0.00005-6
Smith AM, Moxon S, Morris GA (2016) Biopolymers as wound healing materials. In: Ågren MS (ed) Wound healing biomaterials, vol 2. Elsevier Ltd., pp 261–287. https://doi.org/10.1016/B978-1-78242-456-7.00013-1
Alven S, Nqoro X, Aderibigbe BA (2020) Polymer-based materials loaded with curcumin for wound healing applications. Polymers (Basel) 12:1–25. https://doi.org/10.3390/polym12102286
Babaremu K, Oladijo OP, Akinlabi E (2023) Biopolymers: A suitable REPLACEMENT for plastics in product packaging. Adv Ind Eng Polym Res. https://doi.org/10.1016/j.aiepr.2023.01.001
Nissola C, Marchioro MLK, de Souza Leite Mello EV et al (2021) Hydrogel containing (1 → 6)-β-D-glucan (lasiodiplodan) effectively promotes dermal wound healing. Int J Biol Macromol 183:316–330. https://doi.org/10.1016/j.ijbiomac.2021.04.169
Madduma-Bandarage USK, Madihally SV (2021) Synthetic hydrogels: Synthesis, novel trends, and applications. J Appl Polym Sci 138:1–23. https://doi.org/10.1002/app.50376
Ćorković I, Pichler A, Šimunović J, Kopjar M (2021) Hydrogels: Characteristics and application as delivery systems of Phenolic and aroma compounds. Foods 10. https://doi.org/10.3390/foods10061252
Al-Shamkhee D, Al-Aasam AB, Al-Waeli AHA, Abusaibaa GY, Moria H (2022) Passive cooling techniques for ventilation: an updated review. Renew Energy Environ Sustain 7:23. https://doi.org/10.1051/rees/2022011
Ng FSF, Muthu SS, Li Y, Hui PCL (2013) A critical review on life cycle assessment studies of diapers. Crit Rev Environ Sci Technol 43:1795–1822. https://doi.org/10.1080/10643389.2012.671746
Varghese SA, Pulikkalparambil H, Promhuad K et al (2023) Renovation of agrowaste for sustainable food packaging: a review. Polymers (Basel) 15:648. https://doi.org/10.3390/polym15030648
Arzami AN, Ho TM, Mikkonen KS (2022) Valorization of cereal by-product hemicelluloses: Fractionation and purity considerations. Food Res Int 151:110818. https://doi.org/10.1016/j.foodres.2021.110818
Mensah MB, Jumpah H, Boadi NO et al (2021) Assessment of quantities and composition of corn stover in Ghana and their conversion into bioethanol. Sci African 12:e00731. https://doi.org/10.1016/j.sciaf.2021.e00731
Tufail T, Saeed F, Afzaal M et al (2021) Wheat straw: A natural remedy against different maladies. Food Sci Nutr:2335–2344. https://doi.org/10.1002/fsn3.2030
Pakizeh M, Moradi A, Ghassemi T (2021) Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur Polym J 159:110709. https://doi.org/10.1016/j.eurpolymj.2021.110709
Boone L, Santa R, Martins C et al (2017) Preparation and characterization of chitosan obtained from shells of shrimp (litopenaeus vannamei boone). Mar Drug 15:1–12. https://doi.org/10.3390/md15050141
Tye YY, Saurabh CK, Peng LC (2017) Biodegradable polymer films from seaweed polysaccharides: A review on cellulose as a reinforcement material. eXPRESS Polym Lett 11:244–265. https://doi.org/10.3144/expresspolymlett.2017.26
Mahmud MA, Anannya FR (2021) Sugarcane bagasse - A source of cellulosic fiber for diverse applications. Heliyon 7:e07771. https://doi.org/10.1016/j.heliyon.2021.e07771
Melesse GT, Hone FG, Mekonnen MA (2022) Extraction of cellulose from sugarcane bagasse optimization and characterization. Adv Mater Sci Eng 2022:1–10. https://doi.org/10.1155/2022/1712207
Kapoor M, Panwar D, Kaira GS (2016) Bioprocesses for enzyme production using agro-industrial wastes: technical challenges and commercialization potential. In: Dhillon GS, Kaur S (eds) agro-industrial wastes as feedstock for enzyme production. Elsevier Inc, pp 61–93. https://doi.org/10.1016/B978-0-12-802392-1.00003-4
Hogan KJ, Mikos AG (2020) Biodegradable thermoresponsive polymers: Applications in drug delivery and tissue engineering. Polymer (Guildf) 211:123063. https://doi.org/10.1016/j.polymer.2020.123063
Aly AA, Ahmed MK (2021) Nanofibers of cellulose acetate containing ZnO nanoparticles/graphene oxide for wound healing applications. Int J Pharm 598:120325. https://doi.org/10.1016/j.ijpharm.2021.120325
Zhang K, Sun P, Liu H et al (2016) Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr Polym 138:237–243. https://doi.org/10.1016/j.carbpol.2015.11.038
Senapitakkul V, Vanitjinda G, Torgbo S et al (2020) Pretreatment of cellulose from sugarcane bagasse with xylanase for improving dyeability with natural dyes. ACS Omega 5:28168–28177. https://doi.org/10.1021/acsomega.0c03837
Amirah N, Razali M, Sohaimi RM et al (2022) Comparative study on extraction of cellulose fiber from rice straw waste from chemo-mechanical and pulping method. Polymers (Basel) 14:387
Shen X, Shamshina JL, Berton P et al (2015) Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem 18:53–75. https://doi.org/10.1039/c5gc02396c
Plermjai K, Boonyarattanakalin K, Mekprasart W et al (2018) Extraction and characterization of nanocellulose from sugarcane bagasse by ball-milling-assisted acid hydrolysis. AIP Conf Proc 2010. https://doi.org/10.1063/1.5053181
Sun JX, Sun XF, Zhao H et al (2004) Isolation and characterization of cellulose from sugarcane bagasse. Polym Degrad Stab 84:331–339. https://doi.org/10.1016/j.polymdegradstab.2004.02.008
Nkosivele Z, Mzimela T, Linganiso LZ et al (2018) Comparison of cellulose extraction from sugarcane bagasse through alkali 3. Results and discussion. Mater Res 21:1–7
Gond RK, Gupta MK, Jawaid M (2021) Extraction of nanocellulose from sugarcane bagasse and its characterization. Polym Compos 42:5400–5412. https://doi.org/10.1002/pc.26232
Ahmadi S, Hivechi A, Bahrami SH, Milan PB, Sara S (2021) Cinnamon extract loaded electrospun chitosan/gelatin membrane with antibacterial activity. Int J Biol Macromol 173:580–590. https://doi.org/10.1016/j.ijbiomac.2021.01.156
Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 2015:1133–1174. https://doi.org/10.3390/md13031133
Varun TK, Senani S, Jayapal N (2017) Extraction of chitosan and its oligomers from shrimp shell waste , their characterization and antimicrobial effect. Vet World 10(2):170–175. https://doi.org/10.14202/vetworld.2017.170-175
Kandile NG, Zaky HT, Mohamed MI, Nasr AS, Ali YG (2018) Extraction and characterization of Chitosan from shrimp shells. Open J Org Polym Mater 8:33–42. https://doi.org/10.4236/ojopm.2018.83003
Tokatl K (2018) Optimization of chitin and chitosan production from shrimp wastes and characterization. J Food Process Preserv 42:1–13. https://doi.org/10.1111/jfpp.13494
Zhao D, Huang W, Guo N, Zhang S, Xue C (2019) Two-step separation of chitin from shrimp shells using citric acid and deep eutectic solvents with the assistance of microwave. Polymers 11:409. https://doi.org/10.3390/polym11030409
Xie J, Xie W, Yu J et al (2021) Extraction of chitin from shrimp shell by successive two-step fermentation of exiguobacterium profundum and lactobacillus acidophilus. Front Microbiol 12:1–10. https://doi.org/10.3389/fmicb.2021.677126
Tan YN, Lee PP, Chen WN (2020) Microbial extraction of chitin from seafood waste using sugars derived from fruit waste - stream. AMB Express 10:1–11. https://doi.org/10.1186/s13568-020-0954-7
Singh R, Shitiz K, Singh A (2017) Chitin and Chitosan: biopolymers for wound management. Int Wound J 14:1276–1289. https://doi.org/10.1111/iwj.12797
El KH, Belaabed R, Addaou A, Laajeb A, Lahsini A (2018) Extraction , chemical modification and characterization of chitin and chitosan. BIOMAC 120:1181–1189. https://doi.org/10.1016/j.ijbiomac.2018.08.139
Conzatti G, Chamary S, De GN, Cavalie S, Morent R, Tourrette A (2018) Surface functionalization of plasticized chitosan films through PNIPAM grafting via UV and plasma graft polymerization. Eur Polym J 105:434–441. https://doi.org/10.1016/j.eurpolymj.2018.06.020
Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H (2011) Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv 29:322–337. https://doi.org/10.1016/j.biotechadv.2011.01.005
Wang K, Pan S, Qi Z et al (2020) Recent Advances in chitosan-based metal nanocomposites for wound healing applications. Adv Mater Sci Eng 2020:1–13. https://doi.org/10.1155/2020/3827912
Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ER (2020) Alginate-based composite materials for wound dressing application:A mini review. Carbohydr Polym 236:116025. https://doi.org/10.1016/j.carbpol.2020.116025
Batista MP, Gonçalves VSS, Gaspar FB, Nogueira ID, Matias AA, Gurikov P (2020) Novel alginate-chitosan aerogel fibres for potential wound healing applications. Int J Biol Macromol 156:773–782. https://doi.org/10.1016/j.ijbiomac.2020.04.089
Mohammed A, Rivers A, Stuckey DC, Ward K (2020) Alginate extraction from Sargassum seaweed in the Caribbean region: Optimization using response surface methodology. Carbohydr Polym 245:116419. https://doi.org/10.1016/j.carbpol.2020.116419
Rashtchian M, Hivechi A, Bahrami SH, Milan PB, Simorgh S (2020) Fabricating alginate/poly (caprolactone) nanofibers with enhanced bio-mechanical properties via cellulose nanocrystal incorporation. Carbohydr Polym 233:115873. https://doi.org/10.1016/j.carbpol.2020.115873
Malgarim L, Faccendini A, Rossi S et al (2019) Platelet lysate loaded electrospun scaffolds: Effect of nanofiber types on wound healing. 142:247–257. https://doi.org/10.1016/j.ejpb.2019.06.030
Taemeh MA, Shiravandi A, Korayem MA, Daemi H (2020) Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr Polym 228:115419. https://doi.org/10.1016/j.carbpol.2019.115419
Article R, Michalak I, Detyna J (2019) Methods of extraction, physicochemical properties of alginates and their applications in biomedical field–a review. Open Chem 2019:738–762
Saji S, Hebden A, Goswami P, Du C (2022) A brief review on the development of alginate extraction process and its sustainability. Sustainability 14:1–20. https://doi.org/10.3390/su14095181
Trica B, Delattre C, Gros F et al (2019) Extraction and characterization of alginate from an edible brown seaweed (Cystoseira barbata) harvested in the romanian black sea. Mar Drugs 17:1–15. https://doi.org/10.3390/md17070405
Jayasinghe GDTM, Jinadasa BKKK, Sadaruwan NAG (2022) Pathway of sodium alginate synthesis from marine brown algae, Sargassum wightii from Sri Lanka. Discov Food 2:1–7. https://doi.org/10.1007/s44187-021-00001-5
Nat JJ, Prod P, Press I et al (2021) Production of alginate from persian gulf sargassum angustifolium seaweeds : Novel extraction and characterization methods. Nat Pharm Prod 1-10. https://doi.org/10.5812/jjnpp.106011.Research
Dalal SR, Hussein MH, El N, El A, Dessuuki SAS (2021) Characterization of alginate extracted from Sargassum latifolium and its use in Chlorella vulgaris growth promotion and riboflavin drug delivery. Sci Rep 1–17. https://doi.org/10.1038/s41598-021-96202-0
Ahmady A, Hayati N, Samah A (2021) A review: Gelatine as a bioadhesive material for medical and pharmaceutical applications. Int J Pharm 608:121037. https://doi.org/10.1016/j.ijpharm.2021.121037
Alipal J, Pu NASM, Lee TC et al (2021) Materials today: Proceedings A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater Today Proc 42:240–250. https://doi.org/10.1016/j.matpr.2020.12.922
Li R, Wang Y, Li Q, Sun G (2023) Photo-stimuli responsive phosphorescence from carbon dots in porous gelatin. J Lumin 257:119725. https://doi.org/10.1016/j.jlumin.2023.119725
Kommareddy S, Vaccines T, Shenoy D, Amiji M (2007) Gelatin nanoparticles and their biofunctionalization, pp 330–352. https://doi.org/10.1002/9783527610419.ntls0011
Saenmuang S, Phothiset S, Chumnanka C (2020) Extraction and characterization of gelatin from black-bone chicken by-products. Food Sci Biotechnol 29:469–478. https://doi.org/10.1007/s10068-019-00696-4
Chakka AK, Muhammed A, Sakhare PZ, Bhaskar N (2017) Poultry processing waste as an alternative source for mammalian gelatin: Extraction and characterization of gelatin from chicken feet using food grade acids. Waste Biomass Valor 8:2583–2593. https://doi.org/10.1007/s12649-016-9756-1
Rather JA, Majid SD, Dar AH, Amin T (2022) Extraction of gelatin from poultry byproduct: influence of drying method on structural, thermal, functional, and rheological characteristics of the dried gelatin powder. Front Nutr 9:1–14. https://doi.org/10.3389/fnut.2022.895197
Kamatchi P, Leela K (2016) Extraction, characterization and application of gelatin from Carcharhinus amblyrhyncho and Sphyraena barracuda. 2:40–49
Amertaning D, Bachrudin Z, Chin KB et al (2019) characteristics of gelatin extracted from Indonesian local cattle hides using acid and base curing. Pakistan J Nutr 18:443–454. https://doi.org/10.3923/pjn.2019.443.454
Asif M, Al-kahtani HA, Jaswir I, Abutarboush H, Ismail EA (2020) Extraction and characterization of gelatin from camel skin (potential halal gelatin) and production of gelatin nanoparticles. Saudi J Biol Sci 27:1596–1601. https://doi.org/10.1016/j.sjbs.2020.03.022
Buyana B, Alven S, Nqoro X, Aderibigbe BA (2020) Antibiotics encapsulated scaffolds as potential wound dressings. In: Varaprasad K, Vimala K, Rotimi S (eds) Antibiotic materials in healthcare. Elsevier Inc, pp 111–128. https://doi.org/10.1016/b978-0-12-820054-4.00007-0
Mistry PA, Konar MN, Latha S et al (2023) Chitosan superabsorbent biopolymers in sanitary and hygiene applications. Int J Polym Sci 2023:4717905. https://doi.org/10.1155/2023/4717905
Gupta S, Sharma S, Kumar A, Saad M, Husain B, Gupta A (2022) Biopolymers from waste biomass and its applications in the cosmetic industry: A review. Mater Today Proc 68:873–879. https://doi.org/10.1016/j.matpr.2022.06.422
Mondejar-Lopez M, Lopez-Jimenez A, Martínez JCG et al (2022) Comparative evaluation of carvacrol and eugenol chitosan nanoparticles as eco-friendly preservative agents in cosmetics 206:288–297. https://doi.org/10.1016/j.ijbiomac.2022.02.164
Cho C, Kobayashi T (2021) Advanced cellulose cosmetic facial masks prepared from Myanmar thanaka heartwood. Curr Opin Green Sustain Chem 27:100413. https://doi.org/10.1016/j.cogsc.2020.100413
Gaspar AL, Gaspar AB, Contini LRF et al (2022) Lemongrass (Cymbopogon citratus) -incorporated chitosan bioactive films for potential skincare applications. Int J Pharm 628:122301. https://doi.org/10.1016/j.ijpharm.2022.122301
Ta Q, Ting J, Harwood S et al (2021) Chitosan nanoparticles for enhancing drugs and cosmetic components penetration through the skin. Eur J Pharm Sci 160:105765. https://doi.org/10.1016/j.ejps.2021.105765
Feng C, Yang P, Liu H et al (2021) Bilayer porous polymer for efficient passive building cooling. Nano Energy 85:105971. https://doi.org/10.1016/j.nanoen.2021.105971
Ferber S, Behrens AM, McHugh KJ et al (2018) evaporative cooling hydrogel packaging for storing biologics outside of the cold chain. Adv Healthc Mater 7:10–15. https://doi.org/10.1002/adhm.201800220
Lu Z, Strobach E, Chen N, Ferralis N, Grossman JC (2020) Passive sub-ambient cooling from a transparent evaporation-insulation bilayer. Joule 4:2693–2701. https://doi.org/10.1016/j.joule.2020.10.005
Arslan Bin Riaz M, Nasir MA, Nauman S et al (2021) Passive cooling performance of polyacrylamide hydrogel on wooden and brick houses and effect of nanoparticle integration on its mechanical strength. Plast Rubber Compos 50:340–350. https://doi.org/10.1080/14658011.2021.1898880
Lv T, Sun L, Yang Y, Huang J (2021) Bio-inspired hydrogel with all-weather adhesion, cooling and reusability functions for photovoltaic panels. Sol Energy 216:358–364. https://doi.org/10.1016/j.solener.2021.01.028
Xu L, Sun DW, Tian Y et al (2023) Nanocomposite hydrogel for daytime passive cooling enabled by combined effects of radiative and evaporative cooling. Chem Eng J 457:141231. https://doi.org/10.1016/j.cej.2022.141231
What Causes Water Pollution in Africa? – The Last Well (n.d.) https://thelastwell.org/2019/06/what-causes-water-pollution-in-africa/
Li J, Pogodin S, Turbidity R et al (2021) Treatment of textile wastewater using sodium alginate beads. J Phys Conf Ser. https://doi.org/10.1088/1742-6596/1979/1/012005
Bustos-Terrones YA, Bandala ER, Moeller-Chávez GE (2022) Enhanced biological wastewater treatment using sodium alginate-immobilized microorganisms in a fluidized bed reactor Water Sci Eng 15:125–133. https://doi.org/10.1016/j.wse.2022.02.002
Zhang W, Ou J, Wang B et al (2021) Efficient heavy metal removal from water by alginate-based porous nanocomposite hydrogels: The enhanced removal mechanism and influencing factor insight. J Hazard Mater 418. https://doi.org/10.1016/j.jhazmat.2021.126358
Kim S, Seo AY, Lee TG (2020) Functionalized cellulose to remove surfactants from cosmetic products in wastewater. Carbohydr Polym 236:116010. https://doi.org/10.1016/j.carbpol.2020.116010
Bilici Z, Ozay Y (2021) Investigation of the usage potential of calcium alginate beads functionalized with sodium dodecyl sulfate for wastewater treatment contaminated with waste motor oil. Water Environ Res 93:2623–2636. https://doi.org/10.1002/wer.1613
Buyana B, Aderibigbe BA, Ndinteh DT et al (2020) Alginate-pluronic topical gels loaded with thymol, norfloxacin and ZnO nanoparticles as potential wound dressings. J Drug Deliv Sci Technol 60:101960. https://doi.org/10.1016/j.jddst.2020.101960
Mehrabi A, Karimi A, Mashayekhan S et al (2022) In-situ forming hydrogel based on thiolated chitosan/carboxymethyl cellulose (CMC) containing borate bioactive glass for wound healing. Int J Biol Macromol 222:620–635. https://doi.org/10.1016/j.ijbiomac.2022.09.177
Shah SA, Sohail M, Karperien M et al (2023) Chitosan and carboxymethyl cellulose-based 3D multifunctional bioactive hydrogels loaded with nano-curcumin for synergistic diabetic wound repair. Int J Biol Macromol 227:1203–1220. https://doi.org/10.1016/j.ijbiomac.2022.11.307
Li X, Sha X, Yang H et al (2023) Ultrasonic treatment regulates the properties of gelatin emulsion to obtain high-quality gelatin film. Food Chem X 18:100673. https://doi.org/10.1016/j.fochx.2023.100673
Tajeddin B, Arabkhedri M (2020) Polymer science and innovative applications. Elsevier. https://doi.org/10.1016/B978-0-12-816808-0.00016-0
Zhao X, Tian R, Zhou J, Liu Y (2022) Multifunctional chitosan/grape seed extract/silver nanoparticle composite for food packaging application. Int J Biol Macromol 207:152–160. https://doi.org/10.1016/j.ijbiomac.2022.02.180
Zhai X, Zhou S, Zhang R et al (2022) Antimicrobial starch/poly(butylene adipate-coterephthalate) nanocomposite films loaded with a combination of silver and zinc oxide nanoparticles for food packaging. Int J Biol Macromol 206:298–305. https://doi.org/10.1016/j.ijbiomac.2022.02.158
Liu Y, Wang R, Wang D et al (2022) Development of a food packaging antibacterial hydrogel based on gelatin, chitosan, and 3-phenyllactic acid for the shelf-life extension of chilled chicken. Food Hydrocoll 127:107546. https://doi.org/10.1016/j.foodhyd.2022.107546
Dai Y, Sun Q, Wang W et al (2018) Utilizations of agricultural waste as adsorbent for the removal of contaminants: A review. Chemosphere 211:235–253. https://doi.org/10.1016/j.chemosphere.2018.06.179
Funding
Open access funding provided by Walter Sisulu University. This work was supported by the South African National Energy Development Institute (SANEDI).
Author information
Authors and Affiliations
Contributions
Xhamla Nqoro: conceptualization, investigating, writing of first draft, and editing. Raymond Taziwa: conceptualization, reviewing, and editing. Patricia Popoola: conceptualization, reviewing, and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nqoro, X., Taziwa, R. & Popoola, P. Recent progress in the conversion of agricultural waste into functional materials. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-05044-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05044-5