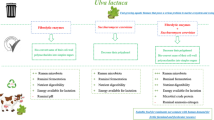
Abstract
Posidonia oceanica wastes is abundant biomass in Mediterranean coasts characterized by a high content of dietary fiber and polyphenolic compounds and pose a serious pollution problem. The aim of this study was to valorize these seagrasses into suitable alternative feed for ruminants through conversion approach with microwaves treatment at a power of 750 W for 240 s and exogenous fibrolytic enzymes (EFE) produced by fermentation of Trichoderma longibrachiatum at a rate of 4 μl/g dry matter for 24 h.
The results showed that microwave treatment improved the amount of rumen fermentation and digestibility of cell wall polysaccharides and dry matter without altering the fermentation rate. The EFE treatments stimulated the rate of rumen fermentation especially in the initial phase and reduced the half-time of fermentation. The effect of EFE became less pronounced with increasing incubation time, as well as did not affect amount of rumen fermentation and digestibility. Only microwave treatment followed by EFE converted some of their cellulose and hemicellulose to reducing sugars. This modification provides a suitable substrate for proliferation of rumen protozoa, accelerates rumen fermentation process, shortens time of onset of rumen fermentation, and increases extent of rumen fermentation. As a result, their digestibility and net energy available for lactation are improved. In addition, this approach stimulates rumen microbiota to produce short-chain fatty acids and to bio-convert rumen ammonia nitrogen to microbial protein. This environmentally friendly process could be used to convert these seagrass wastes into suitable ruminant feeds.
Graphical Abstract

Similar content being viewed by others
Data availability
The datasets and materials used during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- A:
-
Amount of rumen fermentation
- AFR:
-
Average fermentation rate
- C:
-
Rate of fermentation
- EFE:
-
Exogenous fibrolytic enzymes
- GP:
-
Net gas production
- GP24 :
-
Net gas production after 24 h of incubation
- Lag:
-
Time of the onset of fermentation
- SEM:
-
Standard error of means
- t:
-
Incubation time
- T1/2 :
-
Half-time of amount of rumen fermentation
References
Boudouresque CF, Bernard G, Bonhomme P, Charbonnel E, Diviacco G, Meinesz A, Pergen G, Pergent-Martini C, Ruitton S, Tunesi L (2006) Préservation et conservation des herbiers à Posidonia oceanica. Ramoge, Marseille, France
Adamec L (1997) Photosynthetic characteristics of the aquatic carnivorous plant Aldrovanda vesiculosa. Aquat Bot 59(3-4):297–306. https://doi.org/10.1016/S0304-3770(97)00054-5
Shabaka SH, Khalil MK, El-Sikaily A, Youssef NAE (2021) Posidonia oceanica litter along the Mediterranean Coast of Egypt: status and a preliminary assessment of nutrients and trace elements contents. Estuar Coast Shelf Sci 255:107342. https://doi.org/10.1016/j.ecss.2021.107342
Cocozza C, Parente A, Zaccone C, Mininni C, Santamaria P, Miano T (2011) Comparative management of offshore Posidonia residues: composting vs. energy recovery. Waste Manage 31(1):78–84. https://doi.org/10.1016/j.wasman.2010.08.016
Castillo C, Mantecón AR, Sotillo J, Gutiérrez C, Abuelo A, Hernández J (2016) Posidonia oceanica banquettes as a substitute for straw in dairy goat rations: metabolic and productive effects. J Sci Food Agric 96(2):602–609. https://doi.org/10.1002/jsfa.7129
Castillo C, Hernández J, Sotillo Mesanza J, Gutiérrez C, Montes AM, Mantecón ÁR (2018) Effects of Posidonia oceanica banquettes on intake, digestibility, nitrogen balance and metabolic profiles in sheep. J Sci Food Agric 98(7):2658–2664. https://doi.org/10.1002/jsfa.8759
Castillo C, Mantecón AR, Sotillo J, Benedito JL, Abuelo A, Gutiérrez C, Hernández J (2014) The use of banquettes of Posidonia oceanica as a source of fiber and minerals in ruminant nutrition. An observational study. Animal 8(10):1663–1666. https://doi.org/10.1017/S1751731114001505
Abid K, Jabri J, Yaich H, Malek A, Rekhis J, Kamoun M (2022) Nutritional value assessments of peanut hulls and valorization with exogenous fibrolytic enzymes extracted from a mixture culture of Aspergillus strains and Neurospora intermedia. Biomass Convers Biorefin:1–9. https://doi.org/10.1007/s13399-022-03681-w
Abid K, Jabri J, Yaich H, Malek A, Rekhis J, Kamoun M (2022) In vitro study on the effects of exogenic fibrolytic enzymes produced from Trichoderma longibrachiatum on ruminal degradation of olive mill waste. Arch Anim Breed 65:79–88. https://doi.org/10.5194/aab-65-79-2022
Abid K, Jabri J, Yaich H, Malek A, Rekhis J, Kamoun M (2022) Improving the nutritional value and rumen fermentation characteristics of sesame seed coats through bioconversion approach using exogenous fibrolytic enzymes produced by Trichoderma longibrachiatum. Biomass Convers Biorefin:1–9. https://doi.org/10.1007/s13399-022-03402-3
Kholif AE, Gouda GA, Morsy TA, Matloup OH, Fahmy M, Gomaa AS, Patra AK (2022) Dietary date palm leaves ensiled with fibrolytic enzymes decreased methane production, and improved feed degradability and fermentation kinetics in a ruminal in vitro system. Waste Biomass Valor 13:3475–3488. https://doi.org/10.1007/s12649-022-01752-7
Abid K, Jabri J, Ammar H, Ben Said S, Yaich H, Malek A, Rekhis J, Lopez S, Kamoun M (2020) Effect of treating olive cake with fibrolytic enzymes on feed intake, digestibility and performance in growing lambs. Anim Feed Sci Tech 261:114405. https://doi.org/10.1016/j.anifeedsci.2020.114405
Mousa GA, Allak MA, Hassan OGA (2022) Influence of fibrolytic enzymes supplementation on lactation performance of Ossimi ewes. Adv Anim Vet Sci 10(1):27–34. https://doi.org/10.17582/journal.aavs/2022/10.1.27.34
Shishir MSR, Brodie G, Cullen B, Kaur R, Cho E, Cheng L (2020) Microwave heat treatment induced changes in forage hay digestibility and cell microstructure. Appl Sci 10(22):8017. https://doi.org/10.3390/app10228017
Brodie G, Rath C, Devanny M, Reeve J, Lancaster C, Doherty T, Harris G, Chaplin S, Laird C (2012) The effect of microwave treatment on animal fodder. J Microw Power Electromagn Energy 46(2):57–67. https://doi.org/10.1080/08327823.2012.11689824
Dong S, Long R, Zhang D, Hu Z, Pu X (2005) Effect of microwave treatment on chemical composition and in sacco digestibility of wheat straw in yak cow. Asian-australas J Anim Sci 18(1):27–31. https://doi.org/10.5713/ajas.2005.27
Baiely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270. https://doi.org/10.1016/0168-1656(92)90074-J
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. Methods Enzymol 160(87):112. https://doi.org/10.1016/0076-6879(88)60109-1
Association of Official Chemists Analytical Chemists (2000) Official Methods of Analysis, 17th edn. AOAC International, Gaithersburg, MD, USA
Van Soest PV, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74(10):3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France JA (1994) Simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Technol 48(3-4):185–197. https://doi.org/10.1016/0377-8401(94)90171-6
Menke KH, Steingass H (1988) Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev 28:7–55
Mauricio RM, Mould FL, Dhanoa MS, Owen E, Channa KS, Theodorou MK (1999) A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim Feed Sci Technol 79(4):321–330. https://doi.org/10.1016/S0377-8401(99)00033-4
France J, Dijkstra J, Dhanoa MS, Lopez S, Bannink A (2000) Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: derivation of models and other mathematical considerations. Br J Nutr 83:143–150. https://doi.org/10.1017/S0007114500000180
SAS Institute Inc (2011) SAS/STAT 9.3, User’s Guide. SAS Institute Inc, Cary, NC, USA
Dehority BA (1993) Laboratory manual for classification and morphology of rumen ciliate protozoa. CRC Press. https://doi.org/10.1201/9781351073912
Broderick GA, Kang JH (1980) Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci 63:64–75. https://doi.org/10.3168/jds.S0022-0302(80)82888-8
Patra AK, Kamra DN, Agarwal N (2006) Effect of plant extracts on in vitro methanogenesis, enzyme activities and fermentation of feed in rumen liquor of buffalo. Anim Feed Sci Technol 128(3-4):276–291. https://doi.org/10.1016/j.anifeedsci.2005.11.001
Blümmel M, Makkar HPS, Becker K (1997) In vitro gas production: a technique revisited. J Anim Physiol An N 77:24–34. https://doi.org/10.1111/j.1439-0396.1997.tb00734.x
Getachew G, Blummel M, Makkar HPS, Becker K (1998) In vitro gas measuring techniques for assessment of nutritional quality of feeds: a review. Anim Feed Sci Tech 72:261–281. https://doi.org/10.1016/S0377-8401(97)00189-2
Torbatinejad NM, Annison G, Rutherfurd-Markwick K, Sabine JR (2007) Structural constituents of the seagrass Posidonia australis. J Agric Food Chem 55(10):4021–4026. https://doi.org/10.1021/jf063061a
National Research Council (2001) Nutrient requirements of dairy cattle, 7th edn. National Academies Press, Washington, USA. https://doi.org/10.17226/9825
Van Soest PJ (1994) Nutritional ecology of the ruminant, 2nd edn. Cornell University Press, Ithaca, NY
Jabri J, Abid K, Yaich H, Malek A, Rekhis J, Kamoun M (2019) Effect of combining exogenous fibrolytics enzymes supplementation with alkali and acid pre-treatments on wheat straw hydrolysis and ruminal fermentation. Indian J Anim Sci 89(7):780–785
Yagoubi Y, Smeti S, Mekki I, Mahouachi M, Atti N (2021) Effects of rosemary distillation residues substitution to oat hay on diet digestibility, metabolic profile and growth performance of Barbarine ewe lambs. Large Anim Rev 27(5):271–277
Bahri A, Joy M, Blanco M, Bertolin JR, Amaraoui M, Rouissi H (2018) Effects of total replacement of soybean meal and corn on ruminal fermentation, volatile fatty acids, protozoa concentration, and gas production. Arch Anim Breed 61(1):123–130. https://doi.org/10.5194/aab-61-123-2018
Cimmino R, Barone C, Claps S, Varricchio E, Rufrano D, Caroprese M, Albenzio M, De Palo P, Campanile G, Neglia G (2018) Effects of dietary supplementation with polyphenols on meat quality in Saanen goat kids. BMC Vet Res 14(1):1–11. https://doi.org/10.1186/s12917-018-1513-1
Serra V, Salvatori G, Pastorelli G (2021) Dietary polyphenol supplementation in food producing animals: effects on the quality of derived products. Animals 11(2):401. https://doi.org/10.3390/ani11020401
Bešlo D, Došlić G, Agić D, Rastija V, Šperanda M, Gantner V, Lučić B (2022) Polyphenols in ruminant nutrition and their effects on reproduction. Antioxidants 11(5):970. https://doi.org/10.3390/antiox11050970
Yu Z, Ma H, den Boer E, Wu W, Wang Q, Gao M, Vo DVN, Guo M, Xia C (2022) Effect of microwave/hydrothermal combined ionic liquid pretreatment on straw: rumen anaerobic fermentation and enzyme hydrolysis. Environ Res 205:112453. https://doi.org/10.1016/j.envres.2021.112453
Ran T, Saleem A, Shen Y, Ribeiro G, Beauchemin K, Tsang A, Yang W, McAllister TA (2019) Effects of a recombinant fibrolytic enzyme on fiber digestion, ruminal fermentation, nitrogen balance and total tract digestibility of heifers fed a high forage diet. J Anim Sci 97(8):3578–3587. https://doi.org/10.1093/jas/skz216
Hu ZH, Yue ZB, Yu HQ, Liu SY, Harada H, Li YY (2012) Mechanisms of microwave irradiation pretreatment for enhancing anaerobic digestion of cattail by rumen microorganisms. Appl Energy 93:229–236. https://doi.org/10.1016/j.apenergy.2011.12.015
Vasta V, Daghio M, Cappucci A, Buccioni A, Serra A, Viti C, Mele M (2019) Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J Dairy Sci 102(5):3781–3804. https://doi.org/10.3168/jds.2018-14985
García-Rodríguez J, Ranilla MJ, France J, Alaiz-Moretón H, Carro MD, López S (2019) Chemical composition, in vitro digestibility and rumen fermentation kinetics of agro-industrial by-products. Animals 9(11):861. https://doi.org/10.3390/ani9110861
Díaz A, Ranilla MJ, Giraldo LA, Tejido ML, Carro MD (2015) Treatment of tropical forages with exogenous fibrolytic enzymes: effects on chemical composition and in vitro rumen fermentation. J Anim Physiol Anim Nutr (Berl) 99(2):345–355. https://doi.org/10.1111/jpn.12175
Ranilla MJ, Tejido ML, Giraldo LA, Tricárico JM, Carro MD (2008) Effects of an exogenous fibrolytic enzyme preparation on in vitro ruminal fermentation of three forages and their isolated cell walls. Animal Anim Feed Sci Tech 145(1-4):109–121. https://doi.org/10.1016/j.anifeedsci.2007.05.046
Morgavi DP, Beauchemin KA, Nsereko VL, Rode LM, McAllister TA, Wang Y (2004) Trichoderma enzymes promote Fibrobacter succinogenes S85 adhesion to, and degradation of, complex substrates but not pure cellulose. J Sci Food Agric 84(10):1083–1090. https://doi.org/10.1002/jsfa.1790
Lopez S (2005) In vitro and in situ techniques for estimatingdigestibility. In: Dijkstra J, Forbes JM, France J (eds) Quantitative aspects of ruminant digestion and metabolism. CABI Publishing, CAB International, Wallingford, UK, pp 87–122. https://doi.org/10.1079/9780851998145.0000
Li Z, Deng Q, Liu Y, Yan T, Li F, Cao Y, Yao J (2018) Dynamics of methanogenesis, ruminal fermentation and fiber digestibility in ruminants following elimination of protozoa: a meta-analysis. J Anim Sci Biotechnol 9(1):1–9. https://doi.org/10.1186/s40104-018-0305-6
Oba M, Allen MS (1999) Evaluation of the importance of the digestibility of neutral detergent fiber from forage: effects on dry matter intake and milk yield of dairy cows. J Dairy Sci 82(3):589–596. https://doi.org/10.3168/jds.S0022-0302(99)75271-9
Funding
This research was supported by the Laboratory of Animal Nutrition: Management of the Health and Quality of Animal Production [LR14AGR03] (Ministry of Higher Education and Scientific Research, Tunisia).
Author information
Authors and Affiliations
Contributions
Conceptualization, KA and MK. Methodology, KA and MK. Format analyses and investigation, KA, JJ, and HY. Writing draft, KA. Resource, AM, JR, and MK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The article does not contain any studies with human participants. It also does not perform experiments directly on animals. So, this experience does not need ethics statement.
Consent to participate
All the authors of this article are consented to participate.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abid, K., Jabri, J., Yaich, H. et al. Conversion of Posidonia oceanica wastes into alternative feed for ruminants by treatment with microwaves and exogenous fibrolytic enzymes produced by fermentation of Trichoderma longibrachiatum. Biomass Conv. Bioref. 13, 16529–16536 (2023). https://doi.org/10.1007/s13399-023-03830-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03830-9