Abstract
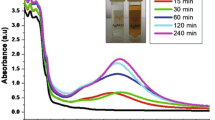
Biofilms are notoriously difficult to eradicate and are a source of many recalcitrant infections. There is effective biofilm management with silver nanoparticles. However, silver nanoparticles that are chemically mediated are extremely harmful to ecosystems. The current study synthesized AgNPs using Cleome rutidosperma leaf extract (CrLE) and analyzed it in UV-VIS, FT-IR, XRD spectroscopy, and TEM for the physical formation and tested with Candida albicans, A431 (Epidermoid carcinoma) cells for its biological efficacy and molecular docking also done. The CrLF has significant phytochemicals such as flavonoids, tannins, terpenoids, and glycosides. The CrLE-AgNPs produced a surface plasmon resonance (SPR) peak at 450 nm in the UV-Vis spectrum and the FT-IR functional groups had -O-H, -N-H stretching at 3423.83 cm-1 due to hydroxyl and amino groups; C=C and C=O stretching at 1632.53 cm-1 due to flavonoid; and C-H deformation at 1110.42 cm-1 due to AgO formation. The XRD peaks revealed the particle size as 14.07 nm. The TEM and SAED studies confirmed the development of spherical shaped AgNPs with agglomeration. During biological characterization, the synthesized AgNPs produced an inhibitory zone against Candida albicans and its biofilm between 30 μg/mL to 50 μg/mL concentrations and produced cytotoxicity against A431 (epidermoid carcinoma) cells (in vitro) at an IC50 value of 36.56 µg/ml. A molecular docking study revealed that a well-conserved binding region is present and predicted the best binding energy value between Als3 adhesin (PDB ID: 4LE8) and silver oxide nanoparticle is -9.60 kcal/mol. The results of this study show that AgNPs made from the leaf extract of Cleome rutidosperma could be used to treat infections caused by anti-candida.










Similar content being viewed by others
References
Vanlalveni C, Lallianrawna S, Biswas Am Selvaraj B, Changmai RSL (2021) Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: a review of recent literature. RSC Adv 11:2804. https://doi.org/10.1039/D0RA09941D
Verma P, Maheshwari S (2019) Applications of silver nanoparticles in diverse sectors. Internat J Nano Dimens 10(1):18–36
Simbine EO, Larissa da CR, Judite L, Lapa-Guimaraes ES, Carlos H, Carlos AF (2019) Application of silver nanoparticles in food packages: a review. Food. Sci Technol 39(4). https://doi.org/10.1590/fst.36318
Almatroudi A (2020) Silver nanoparticles: synthesis, characterisation and biomedical applications. Open Life Science 15(1):819–839. https://doi.org/10.1515/biol-2020-0094
Mathur P, Jha S, Ramteke S, Jain NK (2018) Pharmaceutical aspects of silver nanoparticles. Artif Cells Nanomed Biotechnol 46(sup1):115–126. https://doi.org/10.1080/21691401.2017.1414825
Florencia O, Arce VB, Garcia MA (2021) Nanocomposite starch-based films containing silver nanoparticles synthesized with lemon juice as reducing and stabilizing agent. Carbohydr Polym 252:117208. https://doi.org/10.1016/j.carbpol.2020.117208
Gadkari RR, Ali SW, Alagirusamy R, Das A (2018) Silver nanoparticles in water purification: opportunities and challenges. In: Oves, M., Zain Khan, M., M I Ismail, I. (eds) Modern Age Environmental Problems and their Remediation. Springer, Cham. https://doi.org/10.1007/978-3-319-64501-8_13
Kevin VA, Parthiban TP, Radhasaran R, Madavi SP, Koppole K, Koppole CS (2020) Green synthesized Ag nanoparticles for bio-sensing and photocatalytic applications. ACS Omega 5(22):13123–13129. https://doi.org/10.1021/acsomega.0c01136
Ferdous Z, Nemmar A (2020) Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int J Mol Sci 21(7):2375. https://doi.org/10.3390/ijms21072375
Villaseñor-Basulto DL, Pedavoah MM, Bandala ER (2019) Plant materials for the synthesis of nanomaterials: greener sources. In: Martínez L, Kharissova O, Kharisov B (eds) Handbook of Ecomaterials. Springer, Cham. https://doi.org/10.1007/978-3-319-68255-6_88
Qayyum S, Oves M, Khan AU (2017) Obliteration of bacterial growth and biofilm through ROS generation by facilely synthesized green silver nanoparticles. PLoS One 12(8):e0181363. https://doi.org/10.1371/journal.pone.0181363
Ganesh Kumar A, Sankarganesh P, Parthasarathy V, Bhuvaneswari (2022) Invitro and invivo biological potential of the prepared Feroniella lucida mediated silver nanoparticles. J Sol-Gel Sci Tech 101:411–419. https://doi.org/10.1007/s10971-022-05728-w
Sankarganesh P, Ganesh Kumar A, Parthasarathy V, Baby J, Priyadharsini (2021) Synthesis of Murraya koenigii mediated silver nanoparticles and their invitro and invivo biological potential. J Inorg Organomet Polym Mater 31:2971–2979. https://doi.org/10.1007/s10904-021-01894-6
Donga S, Chanda S (2021) Facile green synthesis of silver nanoparticles using Mangifera indica seed aqueous extract and its antimicrobial, antioxidant and cytotoxic potential (3-in-1 system). Artificial cell, Nanomed, and Biotechnol 49(1):292–302. https://doi.org/10.1080/21691401.2021.1899193
Chinnasamy G, Chandrasekharan S, Koh TW, Bhatnagar S (2021) Synthesis, characterization, antibacterial and wound healing efficacy of silver nanoparticles from Azadirachta indica. Front Microbiol 12:611560. https://doi.org/10.3389/fmicb.2021.611560
Yusuf M (2018) Silver nanoparticles: synthesis and applications. Handbook of Ecomaterials 2343–56. https://doi.org/10.1007/978-3-319-68255-6_16
Oves M, Aslam M, Rauf MA, Qayyum S, Qari HA, Khan MS, Alam MZ, Tabrez S, Pugazhendhi A, Ismail IMI (2018) Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mat Sci & Engineer C, Mat for Biologica Applicat 89:429–443. https://doi.org/10.1016/j.msec.2018.03.035
Oves M, Rauf MA, Aslam M, Qari HA, Sonbol H, Ahmad I, Zaman GS, Saeed M (2022) Green synthesis of silver nanoparticles by Conocarpus Lancifolius plant extract and their antimicrobial and anticancer activities. Sau J Biol Science 29(1):460–471. https://doi.org/10.1016/j.sjbs.2021.09.007
Javed R, Zia M, Naz S et al (2020) Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: recent trends and future prospects. J Nanobiotechnol 18:172. https://doi.org/10.1186/s12951-020-00704-4
Aboyewa JA, Sibuyi NRS, Meyer M, Oguntibeju OO (2021) Green synthesis of metallic nanoparticles using some selected medicinal plants from Southern Africa and their biological applications. Plants 10(9):1929. https://doi.org/10.3390/plants10091929
Masum MMI, Siddiqa MM, Ali KA, Zhang Y, Abdallah Y, Ibrahim E, Qiu W, Yan C, Li B (2019) Biogenic synthesis of silver nanoparticles using Phyllanthus emblica fruit extract and its inhibitory action against the pathogen Acidovorax oryzae strain RS-2 of rice bacterial brown stripe. Front Microbiol 10:820. https://doi.org/10.3389/fmicb.2019.00820
Jain S, Mehata MS (2017) Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep 7:15867. https://doi.org/10.1038/s41598-017-15724-8
Mohanta YK, Biswas K, Jena SK, Hashem A, Abd Allah EF, Mohanta TK (2020) Anti-biofilm and antibacterial activities of silver nanoparticles synthesized by the reducing activity of phytoconstituents present in the Indian medicinal plants. Front Microbiol 11:1143. https://doi.org/10.3389/fmicb.2020.01143
Pirtarighat S, Ghannadnia M, Baghshahi S (2019) Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J Nanostruct Chem 9:1–9. https://doi.org/10.1007/s40097-018-0291-4
Ahamad I, Bano F, Anwer R, Srivastava P, Kumar R, Fatma T (2022) Antibiofilm activities of biogenic silver nanoparticles against Candida albicans. Front Microbiol 12:741493. https://doi.org/10.3389/fmicb.2021.741493
Kean R, Delaney C, Sherry L, Borman A, Johnson EM, Richardson MD, Rautemaa-Richardson R, Williams C, Ramage G (2018) Transcriptome assembly and profiling of Candida auris reveals novel insights into biofilm-mediated resistance. MSphere 3(4):e00334–e00318. https://doi.org/10.1128/mSphere.00334-18
Malik MA, Batterjee MG, Kamli MR, Alzahrani KA, Danish EY, Nabi A (2022) Polyphenol-capped biogenic synthesis of noble metallic silver nanoparticles for antifungal activity against Candida auris. J Fungi 8(6):639. https://doi.org/10.3390/jof8060639
Kamli MR, Alzahrani EA, Albukhari SM, Ahmad A, Sabir JSM, Malik MA (2022) Combination effect of novel bimetallic Ag-Ni nanoparticles with fluconazole against Candida albicans. J Fungi 8:733. https://doi.org/10.3390/jof8070733
Kamli MR, Malik MA, Lone SA, Sabir JSM, Mattar EH, Ahmad A (2021a) Beta vulgaris assisted fabrication of novel Ag-Cu bimetallic nanoparticles for growth inhibition and virulence in Candida albicans. Pharmaceutics 13(11):1957. https://doi.org/10.3390/pharmaceutics13111957
Kamli MR, Srivastava V, Hajrah NH, Sabir JSM, Ali A, Malik MA, Ahmad A (2021b) Phytogenic fabrication of Ag–Fe bimetallic nanoparticles for cell cycle arrest and apoptosis signaling pathways in Candida auris by generating oxidative stress. Antioxidants. 10(2):182. https://doi.org/10.3390/antiox10020182
Khuntia A, Martorell M, Ilango K, Bungau SG, Radu AF, Behl T, Sharifi-Rad J (2022) Theoretical evaluation of Cleome species' bioactive compounds and therapeutic potential: a literature review. Biomed Pharmacother 151:113161
Zarghami Moghaddam P, Mohammadi A, Alesheikh P, Feyzi P, Haghbin A, Mollazadeh S, Sabeti Z, Nakhlband A, Kasaian J (2021) Antibacterial, antifungal, and antioxidant activity of Cleome coluteoides: an in vitro comparative study between leaves, stems, and flowers. Turk J Pharm Sci 18(1):10–16. https://doi.org/10.4274/tjps.galenos.2019.59320
Lakshmanan G, Sathiyaseelan A, Kalaichelvan PT (2018) Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: assessment of their antibacterial and anticancer activity. Karbala Interna J Moder Sci 4:61–68. https://doi.org/10.1016/j.kijoms.2017.10.007
Vijayalakshmi T, Rajeswari P (2016) The Evaluation of the virulence factors of clinical Candida isolates and the anti-biofilm activity of Elettaria cardamomum against multi-drug resistant Candida albicans. Curr Med Mycol 2(2):8–15
Judan Cruz KG, Alfonso ED, Fernando SID, Watanabe K (2022) Candida albicans biofilm inhibition by ethnobotanicals and ethnobotanically-synthesized gold nanoparticles. Front Microbiol 12:665113. https://doi.org/10.3389/fmicb.2021.665113
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55e63
Nguyen A-G, Le HTT, RakeshVerma D-LV, Park C-J (2022) Boosting sodium-ion battery performance using an antimony nanoparticle self-embedded in a 3D nitrogen-doped carbon framework anode. Chem Eng J 429:132359. https://doi.org/10.1016/j.cej.2021.132359
Liu Y, Filler SG (2011) Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot Cell 10(2):168–173. https://doi.org/10.1128/EC.00279-10
Whitesell L, Robbins N, Huang DS, McLellan CA, Shekhar-Guturja T, LeBlanc EV, Nation CS, Hui R, Hutchinson A, Collins C, Chatterjee S, Trilles R, Xie JL, Krysan DJ, Lindquist S, Porco JA, Tatu U, Brown LE, Pizarro J, Cowen LE (2019) Structural basis for species-selective targeting of Hsp90 in a pathogenic fungus. Nat Commun 10:402. https://doi.org/10.1038/s41467-018-08248-w
Sidhu AK, Verma N, Kaushal P (2022) Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front Nanotechnol 3:801620. https://doi.org/10.3389/fnano.2021.801620
Pradeep M, Kruszka D, Kachlicki P, Mondal D, Franklin G (2022) Uncovering the phytochemical basis and the mechanism of plant extract-mediated eco-friendly synthesis of silver nanoparticles using ultra-performance liquid chromatography coupled with a photodiode array and high-resolution mass spectrometry. ACS Sustain Chem Eng 10:562–571. https://doi.org/10.1021/acssuschemeng.1c06960
Sharma D, Suvardhan K, Krishna (2019) Biogenic synthesis of nanoparticles: a review. Arab J Chem 12(8): 3576-3600. https://doi.org/10.1016/j.arabjc.2015.11.002
Karthik C, Suresh S, Sneha Mirulalini G, Kavitha S (2020) A FTIR approach of green synthesized silver nanoparticles by Ocimum sanctum and Ocimum gratissimum on mung bean seeds. Inorgan and Nano-Metal Chem 50(8):606–612. https://doi.org/10.1080/24701556.2020.1723025
Balashanmugam P, Kalaichelvan PT (2015) Biosynthesis characterization of silver nanoparticles using Cassia roxburghii DC. aqueous extract, and coated on cotton cloth for effective antibacterial activity. Internat. J Nanomed 10(Suppl 1):87–97. https://doi.org/10.2147/IJN.S79984
Pallavi SS, Rudayni HA, Bepari A, Niazi SK, Nayaka S (2022) Green synthesis of Silver nanoparticles using Streptomyces hirsutus strain SNPGA-8 and their characterization, antimicrobial activity, and anticancer activity against human lung carcinoma cell line A549. Sau J Biolog Sci 29(1):228–238. https://doi.org/10.1016/j.sjbs.2021.08.084
Mikhailova EO (2020) Silver nanoparticles: mechanism of action and probable bio-application. J Function Biomaterial 11(4):84. https://doi.org/10.3390/jfb11040084
Ali EM, Abdallah BM (2020) Effective inhibition of candidiasis using an eco-friendly leaf extract of Calotropis-gigantean-mediated silver nanoparticles. Nanomaterial (Basel, Switzerland) 10(3):422. https://doi.org/10.3390/nano10030422
Oves M, Qari HA, Felemban NM, Khan MZ, Rehan ZA, Ismail IMI (2017) Marinobacter lipolyticus from Red Sea for lipase production and modulation of silver nanomaterials for anti-candidal activities. IET Nanobiotech 11(4):403–410. https://doi.org/10.1049/iet-nbt.2016.0104
Lakshmanan G, Sathiyaseelan A, Kalaichelvan PT, Murugesan K (2017) Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: assessment of their antibacterial and anticancer activity. Karbala Internat J Modern Sci 4:61e68. https://doi.org/10.1016/j.kijoms.2017.10.007
Calderone RA, Fonzi WA (2001) Virulence factors of Candida albicans. Trends Microbiol 9(7):327–335. https://doi.org/10.1016/s0966-842x(01)02094-7
Liu Y, Filler SG (2022) Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot Cell 10(2). https://doi.org/10.1128/EC.00279-10
Teresa ROM, Nicole R, Leah EC (2017) The Hsp90 chaperone network modulates Candida virulence traits. Trends Microbiol 25(10):809–819. https://doi.org/10.1016/j.tim.2017.05.003
Xie Y, Zhi YL, Jiao X, Feng Z, Jialin S, Zhu Q, Jin S et al (2020) Orchestrated actin nucleation by the Candida albicans polarisome complex enables filamentous growth. J Biol Chem 295(44):14840–14854
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomedicine 12:1227–1249. https://doi.org/10.2147/IJN.S121956
Lara HH, Romero-Urbina DG, Pierce C et al (2015) Effect of silver nanoparticles on Candida albicans biofilms: an ultrastructural study. J Nanobiotechnol 13:91. https://doi.org/10.1186/s12951-015-0147-8
Muthusamy C, Ashokkumar M, Boopathyraja A, Priya VV (2022) Enhanced ferro magnetism of (Cu, Fe/Mn) dual doped ZnO nanoparticles and assessment of in-vitro cytotoxicity and antimicrobial activity for magnetically guided immunotherapy and hyperthermia applications. Vacuum 205:111400
Ashokkumar M, Muthusamy C (2022) Role of ionic radii and electronegativity of co-dopants (Co, Ni and Cr) on properties of Cu doped ZnO and evaluation of In-vitro cytotoxicity. Surface and Interface 30:101968. https://doi.org/10.1016/j.surfin.2022.101968
Beema SRM, Seema S, Alagu LS et al (2022) In vitro and in vivo antibiofilm potential of eicosane against Candida albicans. Appl Biochem Biotechnol 194:4800–4816. https://doi.org/10.1007/s12010-022-03984-8
Harikrishnan P, Arayambath B, Jayaraman VK et al (2022) Thidiazuron, a phenyl-urea cytokinin, inhibits ergosterol synthesis and attenuates biofilm formation of Candida albicans. World J Microbiol Biotechnol 38:224. https://doi.org/10.1007/s11274-022-03410-5
Swoboda RK, Bertram G, Budge S, Gooday GW, Gow NA, Brown AJ (1996) Structure and regulation of the HSP90 gene from the pathogenic fungus Candida albicans. Infect Immun 63(11):4506–4514
Glomb O, Bareis L, Johnsson N (2019) YFR016c/Aip5 is part of an actin nucleation complex in yeast. Biol Open 8:bio044024. https://doi.org/10.1242/bio.044024
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization, draft preparation, writing, and review editing: Ganesh Kumar A and Sankarganesh P; investigation: Pugazhenthi G. Kavya, E, Lokesh E, and Muthusamy C; review editing: Rajasekaran M and Ameer Khusro. All authors have completely read and agreed to the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ganesh Kumar A, Pugazhenthi E, Sankarganesh P et al. “Cleome rutidosperma leaf extract mediated biosynthesis of silver nanoparticles and anti-candidal, anti-biofilm, anti-cancer, and molecular docking analysis”. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03806-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03806-9