Abstract
In recent years, the buildup of antibiotic residues in freshwater and wastewater systems has become a significant environmental problem. The fluorinated quinolone-based drug ciprofloxacin (CIP) is a persistent organic pollutant in freshwater and wastewater streams with broad spectrum antibacterial action. Prosopis juliflora is an environmentally invasive weed that has invaded India’s thousands of hectares of land. In the present investigation, Prosopis juliflora biochar (PJB) was prepared by pyrolysis at a temperature of 550 °C under inert conditions, and the prepared biochar was activated with ZnCl2. The adsorption data of CIP onto synthesized activated biochar was then examined by fitting to adsorption isotherm and kinetic models. The effects of different adsorption parameters (pH, adsorbent dosage, temperature, and initial CIP concentration) were investigated. Prepared PJB-AC had a specific surface area of 360.5 m2/g. The isotherm model fitting results showed that the PJB-AC exhibited a comparable adsorption performance (158.2 mg/g) compared to commercially available activated carbon (147.5 mg/g) towards CIP removal. Kinetic regression results showed that the CIP adsorption was well described by a pseudo-second-order kinetic model indicating chemical adsorption of CIP. This is the first study that reported on understanding the adsorption characteristics of various forms of Prosopis julifora biomass (PJ, PJB, and PJB-AC) on CIP removal from aqueous solutions. The results from this study indicate that PJB-AC is a promising adsorbent for CIP removal.
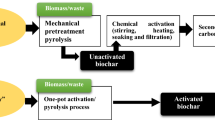
Graphical Abstract









Similar content being viewed by others
References
Mekhamer W, Al-Tamimi S (2019) Removal of ciprofloxacin from simulated wastewater by pomegranate peels. Environ Sci Pollut Res 26:2297–2304. https://doi.org/10.1007/s11356-018-3639-x
Oldenkamp R, Beusen AHW, Huijbregts MAJ (2019) Aquatic risks from human pharmaceuticals - modelling temporal trends of carbamazepine and ciprofloxacin at the global scale. Environ Res Lett 14. https://doi.org/10.1088/1748-9326/ab0071
Subedi B, Balakrishna K, Joshua DI, Kannan K (2017) Mass loading and removal of pharmaceuticals and personal care products including psychoactives, antihypertensives, and antibiotics in two sewage treatment plants in southern India. Chemosphere 167:429–437. https://doi.org/10.1016/j.chemosphere.2016.10.026
Shankar PR, Piryani RM, Piryani S (2017) The state of the world’s antibiotics 2015. J Chitwan Med Coll 6:68–69. https://doi.org/10.3126/jcmc.v6i4.16721
Schaider LA, Rodgers KM, Rudel RA (2017) Review of organic wastewater compound concentrations and removal in onsite wastewater treatment systems. Environ Sci Technol 51:7304–7317. https://doi.org/10.1021/acs.est.6b04778
Zhang J, Chua QW, Mao F et al (2019) Effects of activated carbon on anaerobic digestion – methanogenic metabolism, mechanisms of antibiotics and antibiotic resistance genes removal. Bioresour Technol Reports 5:113–120. https://doi.org/10.1016/j.biteb.2019.01.002
Yang Y, Song W, Lin H et al (2018) Antibiotics and antibiotic resistance genes in global lakes: a review and meta-analysis. Environ Int 116:60–73. https://doi.org/10.1016/j.envint.2018.04.011
Chen Y, Duan X, Zhang C et al (2020) Graphitic biochar catalysts from anaerobic digestion sludge for nonradical degradation of micropollutants and disinfection. Chem Eng J 384:123244. https://doi.org/10.1016/j.cej.2019.123244
Genç N, Dogan EC, Yurtsever M (2013) Bentonite for ciprofloxacin removal from aqueous solution. Water Sci Technol 68:848–855. https://doi.org/10.2166/wst.2013.313
Nogueira J, António M, Mikhalev SM et al (2018) Porous carrageenan-derived carbons for efficient ciprofloxacin removal from water. Nanomaterials 8. https://doi.org/10.3390/nano8121004
Ashiq A, Sarkar B, Adassooriya N et al (2019) Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 236. https://doi.org/10.1016/j.chemosphere.2019.124384
Selvasembian R, Gwenzi W, Chaukura N, Mthembu S (2021) Recent advances in the polyurethane-based adsorbents for the decontamination of hazardous wastewater pollutants. J Hazard Mater 417:125960. https://doi.org/10.1016/j.jhazmat.2021.125960
Ma J, Yang M, Yu F, Zheng J (2015) Water-enhanced removal of ciprofloxacin from water by porous graphene hydrogel. Sci Rep 5:1–11. https://doi.org/10.1038/srep13578
Liu WJ, Jiang H, Yu HQ (2019) Emerging applications of biochar-based materials for energy storage and conversion. Energy Environ Sci 12:1751–1779. https://doi.org/10.1039/c9ee00206e
Yurtsever U, Dogăn EC, Genç N (2017) The use of output-dependent data scaling with artificial neural networks and multilinear regression for modeling of ciprofloxacin removal from aqueous solution. J Water Reuse Desalin 7:25–36. https://doi.org/10.2166/wrd.2016.099
Sennu P, Choi HJ, Baek SG et al (2016) Tube-like carbon for Li-ion capacitors derived from the environmentally undesirable plant: Prosopis juliflora. Carbon N Y 98:58–66. https://doi.org/10.1016/j.carbon.2015.10.087
Nair V, Vinu R (2016) Peroxide-assisted microwave activation of pyrolysis char for adsorption of dyes from wastewater. Bioresour Technol 216:511–519. https://doi.org/10.1016/J.BIORTECH.2016.05.070
Angalaeeswari K, IJCS SK (2017) undefined (2017) Utilization of mesquite (Prosopis juliflora) wood biochar for adsorption of nickel ions in aqueous solution. ChemijournalCom 5:687–690
Ngan A, Jia CQ, Tong S-T (2019) Production, characterization and alternative applications of biochar
Rostamian R, Heidarpour M, Mousavi SF, Afyuni M (2015) Characterization and sodium sorption capacity of biochar and activated carbon prepared from rice husk. J Agric Sci Technol 17:1057–1069
Chandrasekaran A, Ramachandran S, Subbiah S (2017) Determination of kinetic parameters in the pyrolysis operation and thermal behavior of Prosopis juliflora using thermogravimetric analysis. Bioresour Technol 233:413–422. https://doi.org/10.1016/J.BIORTECH.2017.02.119
Rangabhashiyam S, Selvaraju N (2015) Adsorptive remediation of hexavalent chromium from synthetic wastewater by a natural and ZnCl2 activated Sterculia guttata shell. J Mol Liq 207:39–49. https://doi.org/10.1016/j.molliq.2015.03.018
Wang B, Gao B, Fang J (2017) Recent advances in engineered biochar productions and applications. Crit Rev Environ Sci Technol 47:2158–2207. https://doi.org/10.1080/10643389.2017.1418580
Hock PE, Zaini MAA (2018) Activated carbons by zinc chloride activation for dye removal – a commentary. Acta Chim Slovaca 11:99–106. https://doi.org/10.2478/acs-2018-0015
Li J, Yu G, Pan L et al (2018) Study of ciprofloxacin removal by biochar obtained from used tea leaves. J Environ Sci (China) 73:20–30. https://doi.org/10.1016/j.jes.2017.12.024
Li R, Wang Z, Guo J et al (2018) Enhanced adsorption of ciprofloxacin by KOH modified biochar derived from potato stems and leaves. Water Sci Technol 77:1127–1136. https://doi.org/10.2166/wst.2017.636
de Oliveira CC, Costa Rodrigues DL, Lima ÉC et al (2019) Kinetic, equilibrium, and thermodynamic studies on the adsorption of ciprofloxacin by activated carbon produced from Jerivá (Syagrus romanzoffiana). Environ Sci Pollut Res 26:4690–4702. https://doi.org/10.1007/s11356-018-3954-2
Li J, Yu G, Pan L et al (2020) Ciprofloxacin adsorption by biochar derived from co-pyrolysis of sewage sludge and bamboo waste. Environ Sci Pollut Res 27:22806–22817. https://doi.org/10.1007/s11356-020-08333-y
Sayin F, Akar ST, Akar T (2021) From green biowaste to water treatment applications: Utilization of modified new biochar for the efficient removal of ciprofloxacin. Sustain Chem Pharm 24:100522. https://doi.org/10.1016/j.scp.2021.100522
Chandrasekaran A, Patra C, Narayanasamy S, Subbiah S (2020) Adsorptive removal of ciprofloxacin and amoxicillin from single and binary aqueous systems using acid-activated carbon from Prosopis juliflora. Environ Res 188:109825. https://doi.org/10.1016/j.envres.2020.109825
Hu Y, Zhu Y, Zhang Y et al (2019) An efficient adsorbent: simultaneous activated and magnetic ZnO doped biochar derived from camphor leaves for ciprofloxacin adsorption. Bioresour Technol 288:1–8. https://doi.org/10.1016/j.biortech.2019.121511
Özer Ç, İmamoğlu M (2022) Removal of ciprofloxacin from aqueous solutions by pumpkin peel biochar prepared using phosphoric acid. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02832-3
Zuo WQ, Chen C, Cui HJ, Fu ML (2017) Enhanced removal of Cd(ii) from aqueous solution using CaCO3 nanoparticle modified sewage sludge biochar. RSC Adv 7:16238–16243. https://doi.org/10.1039/c7ra00324b
Mao Q, Zhou Y, Yang Y et al (2019) Experimental and theoretical aspects of biochar-supported nanoscale zero-valent iron activating H2O2 for ciprofloxacin removal from aqueous solution. J Hazard Mater 380:120848. https://doi.org/10.1016/j.jhazmat.2019.120848
Sivaraman S, Michael Anbuselvan N, Venkatachalam P et al (2022) Waste tire particles as efficient materials towards hexavalent chromium removal: characterisation, adsorption behaviour, equilibrium, and kinetic modelling. Chemosphere 295:133797. https://doi.org/10.1016/j.chemosphere.2022.133797
Selvakumar A, Rangabhashiyam S (2019) Biosorption of Rhodamine B onto novel biosorbents from Kappaphycus alvarezii, Gracilaria salicornia and Gracilaria edulis. Environ Pollut 255:113291. https://doi.org/10.1016/j.envpol.2019.113291
Rangabhashiyam S, Vijayaraghavan K, Jawad AH, Singh P (2021) Sustainable approach of batch and continuous biosorptive systems for praseodymium and thulium ions removal in mono and binary aqueous solutions. Environ Technol Innov 23:101581. https://doi.org/10.1016/j.eti.2021.101581
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Freundlich H (1907) Über die adsorption in Lösungen. Zeitschrift für Phys. Chemie 57 U:385–470. https://doi.org/10.1515/zpch-1907-5723
Al Jaberi FY, Jabbar SM, Jabbar NM (2020) Modeling of adsorption isotherms of oil content through the electrocoagulation treatment of real oily wastewater. AIP Conf Proc 2213. https://doi.org/10.1063/5.0000157
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem Rev 60:235–241. https://doi.org/10.1021/cr60204a006
Chandarana H, Senthil Kumar P, Seenuvasan M, Anil Kumar M (2021) Kinetics, equilibrium and thermodynamic investigations of methylene blue dye removal using Casuarina equisetifolia pines. Chemosphere 285:131480. https://doi.org/10.1016/j.chemosphere.2021.131480
Jovanovic DS (1969) Physical adsorption of gases. I. Isotherms for monolayer and multilayer adsorption. Kolloid-Zeitschrift Zeitschrift Fur Polym 235:1203-+
Jura G, Harkins WD (1944) Surfaces of solids. XI. Determination of the decrease (π) of free surface energy of a solid by an adsorbed film. J Am Chem Soc 66:1356–1362
Elovich SY, Larinov OG (1962) Theory of adsorption from solutions of non electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form,(II) verification of the equation of adsorption isotherm from solutions. Izv Akad Nauk SSSR, Otd Khim Nauk 2:209–216
Fowler RH, Guggenheim EA (1940) Statistical thermodynamics of super-lattices. Proc R Soc London Ser A Math Phys Sci 174:189–206. https://doi.org/10.1098/rspa.1940.0014
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–59
Mbarki F, Othmani A, Kesraoui A, Seffen M (2021) Coupling alternating current and biosorption for the removal of hexavalent chromium. Chem Eng Technol 44:339–348. https://doi.org/10.1002/ceat.202000398
Yihunu EW, Minale M, Abebe S, Limin M (2019) Preparation, characterization and cost analysis of activated biochar and hydrochar derived from agricultural waste: a comparative study. SN Appl Sci 1:1–8. https://doi.org/10.1007/s42452-019-0936-z
Arami-Niya A, Daud WMAW, Mjalli FS (2010) Using granular activated carbon prepared from oil palm shell by ZnCl2 and physical activation for methane adsorption. J Anal Appl Pyrolysis 89:197–203. https://doi.org/10.1016/j.jaap.2010.08.006
Farobie O, Amrullah A, Bayu A et al (2022) In-depth study of bio-oil and biochar production from macroalgae Sargassum sp. via slow pyrolysis. RSC Adv 12:9567–9578. https://doi.org/10.1039/D2RA00702A
Liu L, Chen X, Wang Z, Lin S (2019) Removal of aqueous fluoroquinolones with multi-functional activated carbon (MFAC) derived from recycled long-root Eichhornia crassipes: batch and column studies. Environ Sci Pollut Res 26:34345–34356. https://doi.org/10.1007/s11356-019-06173-z
Sharifpour N, Moghaddam FM, Mardani G, Malakootian M (2020) Evaluation of the activated carbon coated with multiwalled carbon nanotubes in removal of ciprofloxacin from aqueous solutions. Appl Water Sci 10. https://doi.org/10.1007/s13201-020-01229-9
Liu X, Wan Y, Liu P et al (2018) Optimization of process conditions for preparation of activated carbon from waste Salix psammophila and its adsorption behavior on fluoroquinolone antibiotics. Water Sci Technol 77:2555–2565. https://doi.org/10.2166/wst.2018.205
Duan W, Wang N, Xiao W et al (2018) Ciprofloxacin adsorption onto different micro-structured tourmaline, halloysite and biotite. J Mol Liq 269:874–881. https://doi.org/10.1016/j.molliq.2018.08.051
Movasaghi Z, Yan B, Niu C (2019) Adsorption of ciprofloxacin from water by pretreated oat hulls: equilibrium, kinetic, and thermodynamic studies. Ind Crops Prod 127:237–250. https://doi.org/10.1016/j.indcrop.2018.10.051
Manzar MS, Khan G, dos Santos Lins PV et al (2021) RSM-CCD optimization approach for the adsorptive removal of Eriochrome Black T from aqueous system using steel slag-based adsorbent: characterization, Isotherm, Kinetic modeling and thermodynamic analysis. J Mol Liq 339:116714. https://doi.org/10.1016/j.molliq.2021.116714
Arif M, Liu G, Zia ur Rehman M et al (2022) Carbon dioxide activated biochar-clay mineral composite efficiently removes ciprofloxacin from contaminated water - Reveals an incubation study. J Clean Prod 332:130079. https://doi.org/10.1016/j.jclepro.2021.130079
Zhu X, Tsang DCW, Chen F et al (2015) Ciprofloxacin adsorption on graphene and granular activated carbon: kinetics, isotherms, and effects of solution chemistry. Environ Technol (United Kingdom) 36:3094–3102. https://doi.org/10.1080/09593330.2015.1054316
Ekanayake A, Rajapaksha AU, Selvasembian R, Vithanage M (2022) Amino-functionalized biochars for the detoxification and removal of hexavalent chromium in aqueous media. Environ Res 211:113073. https://doi.org/10.1016/j.envres.2022.113073
Yin D, Xu Z, Shi J et al (2018) Adsorption characteristics of ciprofloxacin on the schorl: kinetics, thermodynamics, effect of metal ion and mechanisms. J Water Reuse Desalin 8:350–359. https://doi.org/10.2166/wrd.2017.143
Ahmed MJ, Theydan SK (2014) Fluoroquinolones antibiotics adsorption onto microporous activated carbon from lignocellulosic biomass by microwave pyrolysis. J Taiwan Inst Chem Eng 45:219–226. https://doi.org/10.1016/j.jtice.2013.05.014
Yu F, Ma J, Bi D (2015) Enhanced adsorptive removal of selected pharmaceutical antibiotics from aqueous solution by activated graphene. Environ Sci Pollut Res 22:4715–4724. https://doi.org/10.1007/s11356-014-3723-9
Jafar Ahamed A, Riaz Ahamed K (2008) Preparation and characterization of activated carbon from the Prosopis juliflora plant. Asian J Chem 20:1702–1706
Rajapaksha AU, Selvasembian R, Ashiq A et al (2022) A systematic review on adsorptive removal of hexavalent chromium from aqueous solutions: recent advances. Sci Total Environ 809:152055. https://doi.org/10.1016/j.scitotenv.2021.152055
Chen D, Xie S, Chen C et al (2017) Activated biochar derived from pomelo peel as a high-capacity sorbent for removal of carbamazepine from aqueous solution. RSC Adv 7:54969–54979. https://doi.org/10.1039/c7ra10805b
Othmani A, Kesraoui A, Boada R et al (2019) Textile wastewater purification using an elaborated biosorbent hybrid material (luffa–cylindrica–zinc oxide) assisted by alternating current. Water 11:1326. https://doi.org/10.3390/w11071326
Othmani A, Magdouli S, Senthil Kumar P et al (2022) Agricultural waste materials for adsorptive removal of phenols, chromium (VI) and cadmium (II) from wastewater: a review. Environ Res 204. https://doi.org/10.1016/j.envres.2021.111916
Zhou Y, Liu X, Xiang Y et al (2017) Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution : adsorption mechanism and modelling Corresponding author at : College of Resources and Environment , Hunan. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.08.178
Yan K, Li R, Yang Z et al (2021) Biomass waste-derived porous carbon efficient for simultaneous removal of chlortetracycline and hexavalent chromium. iScience 24:102421. https://doi.org/10.1016/j.isci.2021.102421
Sun Q, Cui Q, Zhang N et al (2019) The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: a review. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.01.161
Carabineiro SAC, Thavorn-Amornsri T, Pereira MFR et al (2012) Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal Today 186:29–34. https://doi.org/10.1016/j.cattod.2011.08.020
Li J, Yu G, Pan L et al (2020) Ciprofloxacin adsorption by biochar derived from co-pyrolysis of sewage sludge and bamboo waste. Environ Sci Pollut Res 27:22806–22817. https://doi.org/10.1007/s11356-020-08333-y
Mogolodi Dimpe K, Nomngongo PN (2019) Application of activated carbon-decorated polyacrylonitrile nanofibers as an adsorbent in dispersive solid-phase extraction of fluoroquinolones from wastewater. J Pharm Anal 9:117–126. https://doi.org/10.1016/j.jpha.2019.01.003
Fu Y, Yang Z, Xia Y et al (2021) Adsorption of ciprofloxacin pollutants in aqueous solution using modified waste grapefruit peel. Energy Sources, Part A Recover Util Environ Eff 43:225–234. https://doi.org/10.1080/15567036.2019.1624877
Yang Z, Xing R, Zhou W, Zhu L (2020) Adsorption characteristics of ciprofloxacin onto g-MoS2 coated biochar nanocomposites. Front Environ Sci Eng 14. https://doi.org/10.1007/s11783-019-1218-0
Huang W, Chen J, Zhang J (2020) Removal of ciprofloxacin from aqueous solution by rabbit manure biochar. Environ Technol 41:1380–1390. https://doi.org/10.1080/09593330.2018.1535628
Afzal MZ, Sun X-F, Liu J et al (2018) Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads. Sci Total Environ 639:560–569. https://doi.org/10.1016/j.scitotenv.2018.05.129
Sousa ÉML, Otero M, Rocha LS et al (2022) Multivariable optimization of activated carbon production from microwave pyrolysis of brewery wastes - application in the removal of antibiotics from water. J Hazard Mater 431. https://doi.org/10.1016/j.jhazmat.2022.128556
Teixeira RA, Lima EC, Benetti AD et al (2022) Composite of methyl polysiloxane and avocado biochar as adsorbent for removal of ciprofloxacin from waters. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-21176-z
Acknowledgements
The authors thank SASTRA Deemed University for providing infrastructure and research facilities for the successful completion of this research work. The authors would also like to thank Mr. N R Rengasamy for generously providing us with raw materials to conduct this research work.
Availability of data and materials
Data available are on reasonable request.
Funding
Authors, Dr. Shanmugam S. R and Subramaniyasharma S, sincerely thank DST for the financial support through INSPIRE grants DST/INSPIRE/04/2017/002528, SASTRA START-UP grant and TRR Grant. The authors gratefully thank the Department of Science & Technology for support through the FIST programme (Grant: SR/FST/ETI-331/2013). Mrs. Bhuvaneswari Veerapandian sincerely thank CSIR for the financial support through the SRF fellowship (Direct SRF/ 09/1095(0061)/2020 EMR-I).
Author information
Authors and Affiliations
Contributions
Subramaniyasharma, S: writing—original draft preparation, methodology, investigation, and formal analysis. Saravanan R. Shanmugam: data curation, conceptualization, supervision, and funding acquisition. Bhuvaneswari, V: visualization and investigation. Ponnusami, V: writing—review and editing, supervision, and data curation. Rangabhashiyam, S.: resources, writing—reviewing and editing, and supervision.
Ethics declarations
Ethical approval
This is not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Subramaniyasharma, S., Shanmugam, S.R., Bhuvaneswari, V. et al. Pyrolysis of an invasive weed Prosopis juliflora wood biomass for the adsorptive removal of ciprofloxacin. Biomass Conv. Bioref. 13, 9435–9450 (2023). https://doi.org/10.1007/s13399-023-03799-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03799-5