Abstract
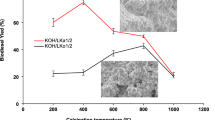
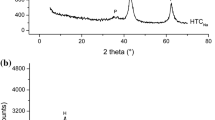
The catalytic behavior of K/Fe catalyst in biodiesel synthesis by ethanolic transesterification of sunflower vegetable oil was investigated. The catalysts were prepared according to the conventional precipitation, impregnation, and calcination methods. The catalysts were characterized by X-ray diffraction (XRD), FT-IR spectroscopy, thermogravimetric analysis (TGA), and Hammett-Benzoic acid indicator titration. Catalytic tests for biodiesel production revealed an excellent activity of the K/Fe3-800 catalyst, which is consistent with the number of basic sites (1.50 mmol/g) in bulk. Potassium polyferrite phases K1.55Fe11O17/K2Fe10O16 as basic active sites on the catalyst surface were generated, increasing the catalytic ability for biodiesel synthesis. The optimum conditions for transesterification reaction were catalyst amount of 5%, ethanol-to-oil molar ratio of 9:1, reaction temperature of 70 °C, and reaction time of 45 min. The maximum conversion of oil to ethyl esters reached 98.2%. The catalyst can tolerate free fatty acid and moisture up to 0.5% and 1%, respectively. K/Fe3-800 catalyst was successfully reused at least three times without post-treatment, and the obtained yield was higher than 92.3%. The XRD and TGA characterizations showed that poisoning of the active sites and pore fouling are the main reasons for the K/Fe3-800 deactivation. This finding indicates that the potassium loading on iron oxide catalysts provides valuable advantages such as low reaction time, easy recovery, and reuse in the transesterification reaction of sunflower oil.
Graphical abstract











Similar content being viewed by others
Data availability
The data could be made available if requested.
Materials availability
The data could be made available if requested.
References
Radwan NRE, Mokhtar M, El-Shobaky GA (2003) Surface and catalytic properties of CuO and Co3O4 solids as influenced by treatment with Co2+ and Cu2+ species. Appl Catal A: Gen 241:77–90. https://doi.org/10.1016/S0926-860X(02)00459-3
Gabal MA, Al-Thabaiti SA, El-Mossalamy EH, Mokhtar M (2010) Structural, magnetic and electrical properties of Ga-substituted NiCuZn nanocrystalline ferrite. Ceram Int 36:1339–1346. https://doi.org/10.1016/j.ceramint.2010.01.021
Ikenaga K, Hamada A, Inoue T, Kusakabe K (2017) Biodiesel production using metal oxide catalysts under microwave heating. Int J Biomass Renew 6:23–26. https://myjms.mohe.gov.my/index.php/ijbr/article/view/4448
Taufiq-Yap YH, Lee HV, Hussein MZ, Yunus R (2011) Calcium-based mixed oxide catalysts for methanolysis of Jatropha curcas oil to biodiesel. Biomass Bioenerg 35:827–834. https://doi.org/10.1016/j.biombioe.2010.11.011
Teo SH, Rashid U, Taufiq-Yap YH (2014) Biodiesel production from crude Jatropha Curcas oil using calcium based mixed oxide catalysts. Fuel 136:244–252. https://doi.org/10.1016/j.fuel.2014.07.062
Hindryawati N, Maniam GP, Karim MdR, Chong KF (2014) Transesterification of used cooking oil over alkali metal (Li, Na, K) supported rice husk silica as potential solid base catalyst. Eng Sci Technol Int J 17:95–103. https://doi.org/10.1016/j.jestch.2014.04.002
Kaur N, Ali A (2015) Biodiesel production via ethanolysis of jatropha oil using molybdenum impregnated calcium oxide as solid catalyst. RSC Adv 5:13285–13295. https://doi.org/10.1039/C4RA14786C
Zhang J, Wang R, Pereira MM (2019) In situ transesterification of microalgae over KOH supported on mesoporous CeO2 catalyst. Pet Coal 61:508–516
Frey PA, Reed GH (2012) The ubiquity of Iron. ACS Chem Biol 7:1477–1481. https://doi.org/10.1021/cb300323q
Post JE (1999) Manganese oxide minerals: crystal structures and economic and environmental significance. Proc Natl Acad Sci USA 96:3447–3454. https://doi.org/10.1073/pnas.96.7.3447
Tang Sh, Wang L, Zhang Y, Li Sh, Tian S, Wang B (2012) Study on preparation of Ca/Al/Fe3O4 magnetic composite solid catalyst and its application in biodiesel transesterification. Fuel Process Technol 95:84–89. https://doi.org/10.1016/j.fuproc.2011.11.022
Dias JM, Alvim-Ferraz MCM, Almeida MF, Diaz JDM, Polo MS, Utrilla JR (2013) Biodiesel production using calcium manganese oxide as catalyst and different raw materials. Energy Convers Manag 65:647–653. https://doi.org/10.1016/j.enconman.2012.09.016
Xue B, Luo J, Zhang F, Fang Z (2014) Biodiesel production from soybean and jatropha oils by magnetic CaFe2O4-Ca2Fe2O5-based catalyst. Energy 68:584–591. https://doi.org/10.1016/j.energy.2014.02.082
Zhang P, Han Q, Fan M, Jiang P (2014) Magnetic solid base catalyst CaO/CoFe2O4 for biodiesel production: influence of basicity and wettability of the catalyst in catalytic performance. Appl Surf Sci 317:1125–1130. https://doi.org/10.1016/j.apsusc.2014.09.043
Liu Y, Zhang P, Fan M, Jiang P (2016) Biodiesel production from soybean oil catalyzed by magnetic nanoparticle MgFe2O4@CaO. Fuel 164:314–321. https://doi.org/10.1016/j.fuel.2015.10.008
Ali MA, Al-Hydary IA, Al-Hattab TA (2017) Nano-magnetic catalyst CaO-Fe3O4 for biodiesel production from date palm seed oil. BCREC 12:460–468. https://doi.org/10.9767/bcrec.12.3.923.460-468
Ali RM, Elkatory MR, Hamad HA (2020) Highly active and stable magnetically recyclable CuFe2O4 as a heterogenous catalyst for efficient conversion of waste frying oil to biodiesel. Fuel 268:117297. https://doi.org/10.1016/j.fuel.2020.117297
Pilorgé E (2020) Sunflower in the global vegetable oil system : situation, specificities and perspectives. OCL 27:34. https://doi.org/10.1051/ocl/2020028
Alptekin E, Canakci M, Sanli H (2014) Biodiesel production from vegetable oil and waste animal fats in a pilot plant. Waste Manag 34:2146–2154. https://doi.org/10.1016/j.wasman.2014.07.019
Shaah MAH, Hossain MdS, Allafi FAS, Alsaedi A, Ismail N, Ab Kadir MO, Ahmad MI (2021) A review on non-edible oil as a potential feedstock for biodiesel : physicochemical properties and production technologies. RSC Adv 11:25018–25037. https://doi.org/10.1039/D1RA04311K
Islam AKMA, Yaakob Z, Anuar N, Primandari SRP (2018) Non-edible vegetable oils as renewable resources for biodiesel production : South-East Asia perspective. In : Nageswara-Rao M and Soneji JR (ed) Advances in biofuels and bioenergy. IntechOpen, United Kingdom, pp 201–215. https://doi.org/10.5772/intechopen.73304
Kombe G, Temu A, Rajabu H, Mrema G, Kansedo J, Lee K (2013) Pre-treatment of high free fatty acids oils by chemical re-esterification for biodiesel production—a review. ACES 3:242–247. https://doi.org/10.4236/aces.2013.34031
Pinto PS, Lanza GD, Ardisson JD, Lago RM (2019) Controlled dehydration of Fe(OH)3 to Fe2O3: developing mesopores with complexing iron species for the adsorption of β-Lactam antibiotics. J Braz Chem Soc 30:310–317. https://doi.org/10.21577/0103-5053.20180179
Ouédraogo WKI, Mouras S, Changotadé OA, Blin J (2018) Development of a new solid catalyst for biodiesel production using local vegetable resources, adapted to the contexts of the West African countries. Waste Biomass Valor 9:1893–1901. https://doi.org/10.1007/s12649-017-9964-3
Zhao X, Su Y, Li S, Bi Y, Han X (2018) A green method to synthesize flowerlike Fe(OH)3 microspheres for enhanced adsorption performance toward organic and heavy metal pollutants. J Environ Sci 73:47–57. https://doi.org/10.1016/j.jes.2018.01.010
Me Nze VM, Fontaine C, Barbier JJr, (2016) Preparation and characterization of MgAlCe mixed oxides for catalytic oxidation of acetic acid. C R Chimie 20:67–77. https://doi.org/10.1016/j.crci.2016.02.018
Dai YM, Hsieh CH, Lin JH, Chen FH, Chen CC (2019) Biodiesel production using bauxite in low-cost solid base catalyst precursors. Catalysts 9:1064. https://doi.org/10.3390/catal9121064
Takase M, Zhang M, Feng W, Chen Y, Zhao T, Cobbina SJ, Yang L, Wu X (2014) Application of zirconia modified with KOH as heterogeneous solid base catalyst to new non-edible oil for biodiesel. Energy Convers Manag 80:117–125. https://doi.org/10.1016/j.enconman.2014.01.034
Rooymans CJM, Langereis C, Schulkes JA (1965) KFe5O8, a new phase in the K2O - Fe2O3 system. Solid State Comm 3:85–87. https://doi.org/10.1016/0038-1098(65)90006-2
Rossetti I, Bencini E, Trentini L, Forni L (2005) Study of the deactivation of a commercial catalyst for ethylbenzene dehydrogenation to styrene. Appl Catal A : Gen 292:118–123. https://doi.org/10.1016/j.apcata.2005.05.046
Legutko P, Jakubek T, Kaspera W, Stelmachowski P, Sojka Z, Kotarba A (2017) Strong enhancement of deSoot activity of transition metal oxides by alkali doping: additive effects of potassium and nitric oxide. Top Catal 60:162–170. https://doi.org/10.1007/s11244-016-0727-3
Zhu XM, Schön M, Bartmann U, van Veen AC, Muhler M (2004) The dehydrogenation of ethylbenzene to styrene over a potassium-promoted iron oxide-based catalyst: a transient kinetic study. Appl Catal A : Gen 266:99–108. https://doi.org/10.1016/j.apcata.2004.02.002
Sievers C, Noda Y, Qi L, Albuquerque EM, Rioux RM, Scott SL (2016) Phenomena affecting catalytic reactions at solid−liquid interfaces. ACS Catal 6:8286–8307. https://doi.org/10.1021/acscatal.6b02532
Fina F, Menard H, Irvine JTS (2015) The effect of Pt NPs crystallinity and distribution on the photocatalytic activity of Pt–g-C3N4. Phys Chem Chem Phys 17:13929. https://doi.org/10.1039/C5CP00560D
Noiroj K, Intarapong P, Luengnaruemitchai A, Jai-In S (2009) A comparative study of KOH/Al2O3 and KOH/NaY catalysts for biodiesel production via transesterification from palm oil. Renew Energy 34:1145–1150. https://doi.org/10.1016/j.renene.2008.06.015
Khanna L, Verma NK (2014) Synthesis, characterization and biocompatibility of potassium ferrite nanoparticles. J Mater Sci Technol 30:30–36. https://doi.org/10.1016/j.jmst.2013.10.008
Li Y, Qiu F, Yang D, Li X, Sun P (2011) Preparation, characterization and application of heterogeneous solid base catalyst for biodiesel production from soybean oil. Biomass Bioenerg 35:2787–2795. https://doi.org/10.1016/j.biombioe.2011.03.009
Feyzi M, Hassankhani A, Rafiee HR (2013) Preparation and characterization of Cs/Al/Fe3O4 nanocatalysts for biodiesel production. Energy Convers Manage 71:62–68. https://doi.org/10.1016/j.enconman.2013.03.022
Lima DFB, dos Santos LF, Pereira DB, Lenzi MK, Corazza ML, Voll FAP (2016) Liquid-liquid equilibrium in systems containing olive oil, free fatty acids, ethanol and water. Open Chem Eng J 10:10–17. https://doi.org/10.2174/1874123101610010010
Ortiz LJ, Martinez DL, Gomez YC, Pfeiffer H (2011) Towards understanding the thermoanalysis of water sorption on lithium orthosilicate (Li4SiO4). Thermochim Acta 515:73–78. https://doi.org/10.1016/j.tca.2010.12.025
Galvez ME, Ascaso S, Stelmachowski P, Legutko P, Kotarba A, Moliner R, Lazaro MJ (2014) Influence of the surface potassium species in Fe–K/Al2O3 catalysts on the soot oxidation activity in the presence of Nox. Appl Catalysis B : Environ 152–153:88–98. https://doi.org/10.1016/j.apcatb.2014.01.041
MacLeod CS, Harvey AP, Lee AF, Wilson K (2008) Evaluation of the activity and stability of alkali-doped metal oxide catalysts for application to an intensified method of biodiesel production. Chem Eng J 135:63–70. https://doi.org/10.1016/j.cej.2007.04.014
Gomez NA, Abonia R, Cadavid H, Vargas IH (2011) Chemical and spectroscopic characterization of a vegetable oil used as dielectric coolant in distribution transformers. J Braz Chem Soc 22:2292–2303. https://doi.org/10.1590/S0103-50532011001200009
Lamberov AA, Dementyeva EV, Vavilov DI, Kuzmina OV, Gilmullin RR, Pavlova EA (2012) The influence of ceric oxide on phase composition and activity of iron oxide catalysts. Adv Chem Eng Sci 2:28–33. https://doi.org/10.4236/aces.2012.21004
Argyle MD, Bartholom CH (2015) Heterogeneous catalyst deactivation and regeneration : a review. ACS Catal 5:145–269. https://doi.org/10.3390/catal5010145
Dejean A, Ouédraogo WKI, Mouras S, Valette J, Blin J (2017) Shea nut shell based catalysts for the production of ethylic biodiesel. Energy Sustain Dev 40:103–111. https://doi.org/10.1016/j.esd.2017.07.006
Hu S, Guan Y, Wang Y, Han H (2011) Nano-magnetic catalyst KF/CaO–Fe3O4 for biodiesel production. Appl Energy 88:2685–2690. https://doi.org/10.1016/j.apenergy.2011.02.012
Macedo AL, Fabris JD, Pires MJM, Oliveira WL, Ardisson JD, Augusti R, Aragón FH, Santos RS, Oliveira LCA, Pereira MC (2016) A mesoporous SiO2/γ-Fe2O3/KI heterogeneous magnetic catalyst for the green synthesis of biodiesel. J Braz Chem Soc 27:2290–2299. https://doi.org/10.5935/0103-5053.20160124
Liu K, Wang R, Yu M (2017) Biodiesel production from soybean oils by a novel nano-magnetic solid base catalyst (K/ZrO2/γ-Fe2O3). RSC Adv 7:51814–51821. https://doi.org/10.1039/C7RA10067A
Kazemifard S, Nayebzadeh H, Saghatoleslami N, Safakish E (2018) Assessment the activity of magnetic KOH/Fe3O4@Al2O3 core–shell nanocatalyst in transesterification reaction : effect of Fe/Al ratio on structural and performance. Environ Sci Pollut Res 25:32811–32821. https://doi.org/10.1007/s11356-018-3249-7
Ghalandari A, Taghizadeh M, Rahmani M (2019) Statistical optimization of the biodiesel production process using a magnetic core-mesoporous shell KOH/Fe3O4@γ-Al2O3 nanocatalyst. Chem Eng Technol 42:89–99. https://doi.org/10.1002/ceat.201700658
Wang YT, Wang XM, Gao D, Wang FP, Zeng YN, Li JG, Jiang LQ, Yu Q, Ji R, Kang LL, Wang YJ, Fang Z (2022) Efficient production of biodiesel at low temperature using highly active bifunctional Na-Fe-Ca nanocatalyst from blast furnace waste. Fuel 322:124168. https://doi.org/10.1016/j.fuel.2022.124168
Acknowledgements
We gratefully acknowledge the Service de Coopération et d’Action Culturelle (SCAC-Burkina) for providing a research facility for this study.
Funding
This research work was conducted under the financial support of The World Academy of Sciences (TWAS), and the European Union (EU) through the PRONOVABIO project.
Author information
Authors and Affiliations
Contributions
Supervision: IWKO; conceptualization: IWKO, JB, and SM; methodology: IWKO, JB, and SM; experiments: IWKO, BW, and EBHT; writing the original draft: IWKO, JB, and SM; writing, review, and editing: IWKO, JB, and SM; funding acquisition: IWKO, JB, and SM.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• K/Fe was prepared and used as basic heterogeneous catalyst for ethanolic transesterification of sunflower oil.
• K/Fe3-800 catalyst achieved 98.2% yield within 45 min of reaction time.
• Potassium polyferrite (K1.55Fe11O17/K2Fe10O16) diffraction peaks were identified as the main basic active sites.
• TGA was used to study the deactivation mechanism and the regeneration of the K/Fe3-800 catalyst.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ouédraogo, I.W.K., Tchuessa, E.B.H., Sawadogo, B. et al. Synthesis of potassium polyferrite KxFeyOz heterogeneous catalyst for sunflower oil ethanolic transesterification. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03773-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03773-1