Abstract
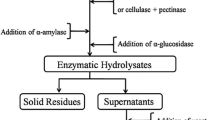
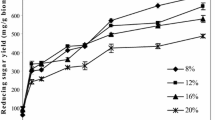
Sweet potato residue (SPR), a by-product of sweet potato starch extraction, is full of starch and cellulose and could be used as the starting material for bioethanol production. A novel one-step complex enzyme (including α-amylase, glucoamylase, cellulase, hemicellulase, and pectinase) hydrolysis approach was developed to liberate the fermentable carbohydrates present in SPR. The effects of pH, amount of enzymes, solid-to-liquid ratio, temperature, and enzyme reaction time on the reducing sugar yield were investigated. Experiment results showed that the optimum pH, solid-to-liquid ratio, amount of enzymes, enzymatic hydrolysis temperature, and time were 4.5, 1: 7, 0.32 g, 50 ℃, and 6 h, respectively. Under these optimum conditions, the experimental reducing sugar yield reached 65.06% ± 1.62%. Carbohydrate analysis of the enzymatic SPR showed that glucose accounted for the largest proportion of fermentable sugars at 58.91% ± 1.25%. In particular, 64.98% ± 0.11% of the cellulose was decomposed during the enzymatic hydrolysis. Finally, a concentration of 113.63 ± 1.35 g/L glucose was formed from the 17.2% (w/v) SPR substrate, and 46.9 ± 0.61 g/L ethanol was finally produced by an industrial diploid Saccharomyces cerevisiae strain at a yield of 27.27% ± 0.30% SPR. The proposed approach has great potential for industrial bioethanol production from SPR due to its high productivity, easy manipulation, and environmentally friendly characteristics.





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Code availability
This is not applicable.
References
Bušić A et al (2018) Bioethanol production from renewable raw materials and its separation and purification: a review. Food Technol Biotechnol 56(3):289–311
Saeed MA et al (2018) Concise review on ethanol production from food waste: development and sustainability. Environ Sci Pollut Res 25(29):28851–28863
Hill J et al (2006) Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci USA 103(30):11206–11210
Banerjee S et al (2010) Commercializing lignocellulosic bioethanol: technology bottlenecks and possible remedies. Biofuels Bioproducts and Biorefining-Biofpr 4(1):77–93
Wang MY et al (2012) Cellulolytic enzyme production and enzymatic hydrolysis for second-generation bioethanol production. Biotechnol China III: Biofuels Bioenergy 128:1–24
Karmee SK (2016) Liquid biofuels from food waste: current trends, prospect and limitation. Renew Sustain Energy Rev 53:945–953
Matsakas L et al (2014) Utilization of household food waste for the production of ethanol at high dry material content. Biotechnol Biofuels 7(1):4
Littlewood J et al (2013) Techno-economic potential of bioethanol from bamboo in China. Biotechnol Biofuels 6(1):173
Xia J et al (2020) Synergism of cellulase, pectinase and xylanase on hydrolyzing differently pretreated sweet potato residues. Prep Biochem Biotechnol 50(2):181–190
Xu C et al (2016) Sweet potato residue as a substrate for microbial fermentation. Feed Industry 37(15):1–6
Yang SS, Ling MY (1989) Tetracycline production with sweet potato residue by solid state fermentation. Biotechnol Bioeng 33(8):1021–1028
Hao ZH et al (2014) Sweet potato starch residue as starting material to prepare polyacrylonitrile adsorbent via SI-SET-LRP. J Agric Food Chem 62(8):1765–1770
Wang FZ et al (2016) An environmentally friendly and productive process for bioethanol production from potato waste. Biotechnol Biofuels 9:50
Zeng X et al (2017) Research progress on fermentation utilization of sweet potato residue. South China Agric 11(34):39–41, 65
Aritonang A et al (2021) The production of citric-acid from Bilimbi (Averrhoa bilimbi) fruits through the fermentation process using Aspergillus niger. IOP Conf. Series: Earth and Environmental Science 782(3):032100
Pagana I, Morawicki R, Hager TJ (2014) Lactic acid production using waste generated from sweet potato processing. Int J Food Sci Technol 49(2):641–649
Nuanpeng S et al (2016) Ethanol production from sweet sorghum juice at high temperatures using a newly isolated thermotolerant yeast Saccharomyces cerevisiae DBKKU Y-53. Energies 9(4):253
Aziz NH, Mohsen GI (2002) Bioconversion of acid- and gamma-ray-treated sweet potato residue to microbial protein by mixed cultures. J Ind Microbiol Biotechnol 29(5):264–267
Yang L, Tan L, Liu T (2021) Progress in detoxification of inhibitors generated during lignocellulose pretreatment. Chin J Biotechnol 37(01):15–29
Ma J et al (2010) Determination of total starch content in feed by 3,5-dinitrosalicylic acid colorimetric method. China feed 14:38–40
Xiao H et al (2012) Metabolic engineering of D-xylose pathway in Clostridium beijerinckii to optimize solvent production from xylose mother liquid. Metab Eng 14(5):569–578
Sluiter JB et al (2021) Direct determination of cellulosic glucan content in starch-containing samples. Cellulose 28(4):1989–2002
An Y et al (2016) Determination of cellulose and hemicellulose content in potato stalk. Mod Agric Sci Technol 17:159–160
Ding J et al (2010) Determination of sisal pectin content by carbazole colorimetric method. Food Res Dev 31(11):138–140
Fang L et al (2020) The determination of moisture, total ash and acid-insoluble ash in fried myrrh. Anhui Chem Ind 46(01):100–102
Yuan J (2013) Optimum digestion temperature for determination of protein content by Kjeldahl method. Cereal Feed Ind 11:25–27
Luo C, Zhang C (2019) Study on hydrolysis of black soybean starch. J Shaanxi Univ Sci Technol 37(01):48–52
Idrees M et al (2013) Optimization of dilute acid pretreatment of water hyacinth biomass for enzymatic hydrolysis and ethanol production. EXCLI J 12:30–40
Ben Taher I et al (2017) Optimization of enzymatic hydrolysis and fermentation conditions for improved bioethanol production from potato peel residues. Biotechnol Prog 33(2):397–406
Li Y et al (2022) Forecasting of reducing sugar yield from corncob after ultrafine grinding pretreatment based on GM(1, N) method and evaluation of biohydrogen production potential. Bioresour Technol 348:126836
Wu Y et al (2014) Studies on technique of producing glucose from sweet potato residue by enzymes. J Agric Sci Technol 16(01):157–162
Wang S et al (2020) Optimization of cellulose hydrolysis from sweet potato residue by cellulase. Food Res Dev 41(08):179–182
Peng Z et al (2013) Optimization of conditions on dilute H2SO4 treatment to improve enzyme decomposition saccharify of lignocellulose from Chinese silvergrass treated by irradiation. J Anhui Agric Univ 41(31):2455–12457, 12461
Xie R, Li M, Long M (2020) The study on selective enzymatic hydrolysis of hemicellulose from Spartina anglica. J Jiangxi Normal Univ ( Nat Sci) 44(05):501–505
Wu X et al (2014) Analysis of the monosaccharide composition of water-soluble polysaccharides from Sargassum fusiforme by high performance liquid chromatography/electrospray ionisation mass spectrometry. Food Chem 145:976–983
Li PP et al (2021) Analysis of monosaccharide composition of water-soluble polysaccharides from Codium fragile by ultra-performance liquid chromatography-tandem mass spectrometry. J Sep Sci 44(7):1452–1460
Han J et al (2009) Analysis of monosaccharide compositions in Heterosmilax japonica polysaccharide by precolumn derivation HPLC. J Chin Med Mat 32(06):893–895
Ma XL et al (2017) Compositional monosaccharide analysis of Morus nigra Linn by HPLC and HPCE quantitative determination and comparison of polysaccharide from Morus nigra Linn by HPCE and HPLC. Curr Pharm Anal 13(5):433–437
Wang X et al (2019) Unraveling the genetic basis of fast l-arabinose consumption on top of recombinant xylose-fermenting Saccharomyces cerevisiae. Biotechnol Bioeng 116(2):283–293
Diao LY et al (2013) Construction of fast xylose-fermenting yeast based on industrial ethanol-producing diploid Saccharomyces cerevisiae by rational design and adaptive evolution. BMC Biotechnol 13:110
Succi M et al (2017) Sub-optimal pH preadaptation improves the survival of Lactobacillus plantarum strains and the malic acid consumption in wine-like medium. Front Microbiol 8:470
Meng J et al (2022) Cloning and characterization of sugar transporter genes of Kluyveromyces marxianus GX-UN120. Microbiol China 1–21
Baks T et al (2008) Effect of gelatinization and hydrolysis conditions on the selectivity of starch hydrolysis with alpha-amylase from Bacillus licheniformis. J Agric Food Chem 56(2):488–495
Zhang JH, Pakarinen A, Viikari L (2013) Synergy between cellulases and pectinases in the hydrolysis of hemp. Biores Technol 129:302–307
Wong DWS et al (2007) Synergistic action of recombinant a-amylase and glucoamylase on the hydrolysis of starch granules. Protein J 26:159–164
Wang J, Xing Y, Zhang L (2022) Optimization of enzymatic hydrolysis process and activity of Korean pine kernel polypeptide. China Oils and Fats 1–15
Frieden E, Walter C (1963) Prevalence and significance of the product inhibition of enzymes. Nature 198:834–837
Funding
This work was supported by the Doctoral Scientific Research Start–up Foundation from Henan University of Technology (No. 2018BS072), the Key scientific research projects of universities in Henan Province (No. 22A180015), and The Open competition Research Projects of Xuchang University (No. 2022JBGS06).
Author information
Authors and Affiliations
Contributions
Chenchen Gou: investigation, writing—original draft preparation, and writing—reviewing and editing. Xiao Wang: formal analysis and writing—original draft preparation. Yuxin Yu: investigation and writing—original draft preparation. Jihong Huang: project administration. Xin Wang: writing—original draft preparation, writing—reviewing and editing, and funding acquisition. Ming Hui: supervision.
Corresponding authors
Ethics declarations
Ethics approval
This paper is new, neither the entire paper nor any part of its content has been published or has been accepted elsewhere. It is not being submitted to any other journal.
Consent to participate
This is not applicable.
Consent for publication
This is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gou, C., Wang, X., Yu, Y. et al. One-step enzymatic hydrolysis of sweet potato residue after gelatinization for bioethanol production by Saccharomyces cerevisiae. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03755-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03755-3