Abstract
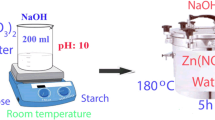
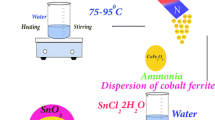
The widespread problem of water pollution is jeopardizing our health. Elimination of dye pollution from the solvent phase by adsorption is a necessary aspect of research. In this research, CuMn2O4/CuMnO nanocomposites were prepared via the co-precipitation method and used to remove dye pollution from an aqueous solution. These nanocomposites were synthesized for the first time in this work. The techniques including XRD, SEM, EDS, VSM, FT-IR, and UV–Vis were used to identify the nanocomposites. The nanocomposites showed a ferromagnetic behavior (coercivity ~ 1082.55 Oe) in low fields and an antiferromagnetic behavior in high fields. Also, their photocatalytic properties for eriochrome black T degradation were studied under visible light irradiation, for the first time. The experiments were repeated in the presence of sucrose as a capping agent. The structure, morphology, and photocatalytic properties of two samples prepared in the presence and absence of a capping agent for eriochrome black T degradation were investigated and compared. The results showed that the sample prepared in the absence of a capping agent has better morphology and photocatalytic activity for the removal of eriochrome black T from an aqueous solution. The photodegradation amount of eriochrome black T solution with 10 ppm concentration by 0.03 g nanocomposite was obtained 76%. The eriochrome black T degradation at acidic pH occurred at a higher rate (80.45% after 90 min) than that at other pHs.











Similar content being viewed by others
References
Buthelezi SP, Olaniran AO, Pillay B (2012) Molecules 17:14260–14274
Rahman QI, Ahmad M, Misra SK, Lohani M (2013) Mater Lett 91:170–174
Crişan M, Drăgan N, Crişan D, Ianculescu A, Niţoi I, Oancea P (2015) Ceram Int 42:3088–3095
Wang S, Hou Y, Wang X, Appl ACS (2015) Mater Interfaces 7:4327–4335
Larbi T, Doll K, Amlouk M (2019) Spectrochim Acta A Mol Biomol Spectrosc 216:117–124
Sinha APB, Sanjana NR, Biswas AB (1958) J Phys Chem B 62:191–194
Radhakrishnan NK, Biswas ABA (1974) J Indian Chem Soc 51:274–280
Verwey EJW, Heilmann EL (1947) J Chem Phys 15:174–180
Enhessari M, Salehabadi A, Maarofian K, Khanahmadzadeh S (2016) Int J Bio-Inorg Hybr Nanomater 5:115–120
Popescu I, Boudjemaa A, Helaili N, Bessekhouad Y, Tudorache M, Bachari K, Marcu IC (2015) Appl Catal A: Gen 504:29–36
Shoemaker DP, Li J, Seshadri R (2009) J Am Chem Soc 131:11450–11457
Saravanakumar B, Muthu Lakshmi S, Ravi G, Ganesh V, Sakunthala A, Yuvakkumar R (2017) J Alloys Compd 723:115–122
Sobhani A (2022) Int J Hydrogen Energy 47:20138–20152
Li H, Chen Y, Ma Q, Wang J, Che Q, Wang G, Tan Y, Yang P (2018) Mater Lett 216:199–202
Wright PA, Natarajan S, Thomas JM, Gai-Boyes PL (1992) Chem Mater 4:1053–1065
Porta P, Moretti G, Musicanti M, Nardella A (1993) Solid State Ionics 63–65:257–267
Koleva V, Stoilova D, Mehandjiev D (1997) J Solid State Chem 133:416–422
Krämer M, Schmidt T, Stöwe K, Maier WF (2006) Appl Catal A 302:257–263
Hlongwa NW, Sastre D, Iwuoha E, Carrillo AJ, Ikpo C, Serrano DP, Pizarro P, Coronado JM (2018) Solid State Ionics 320:316–324
Chani MTS, Karimov KS, BahadarKhan S, Fatima N, Asiri AM (2019) Ceram Int 45:10565–10571
Zhang C, Xie A, Zhang W, Chang J, Liu C, Gu L, Duo X, Pan F, Luo S (2021) J Energy Storage 34:102181–102183
Bayat S, Sobhani A, Salavati-Niasari M (2017) J Mater Sci: Mater Electron 28:16981–16991
Bayat S, Sobhani A, Salavati-Niasari M (2018) J Mater Sci: Mater Electron 29:7077–7089
Mohassel R, Sobhani A, Salavati-Niasari M (2019) Int J Hydrogen Energy 44:860–869
Mohassel R, Amiri M, Kareem Abbas A, Sobhani A, Ashrafi M, Moayedi H, Salavati-Niasari M (2020) J Mater Res Technol 9:1720–1733
Wang LJ, Zhou Q, Liang Y, Shi H, Zhang G, Wang B, Zhang W, Lei B, Wang WZ (2013) Appl Surf Sci 271:136–140
Abel MJ, Pramothkumar A, Senthilkumar N, Jothivenkatachalam K, Inbaraj PFH, Prince J (2019) J Phys B (Amsterdam, Neth.) 572:117–124
Ma P, Geng Q, Gao X, Yang S, Liu G (1973) Ceram Int 42(2016):11966–11971
Mohassel R, Sobhani A, Goudarzi M, Salavati-Niasari M (2018) J Alloys Compd 753:615–621
Mohassel R, Sobhani A, Salavati-Niasari M, Goudarzi M (2018) Spectrochim Acta, Part A 204:232–240
Mahdiani M, Sobhani A, Salavati-Niasari M (2019) J Hazard Mater 367:607–619
Mahdiani M, Sobhani A, Salavati-Niasari M (2017) Sep Purif Technol 185:140–148
Inamdar J, Singh SK (2008) Int J Chem Biomol Eng 1:160–164
Aisien FA, Amenaghawon NA, Ekpenisi EF (2013) J Eng Appl Sci 9:11–16
Sayilkan F, Asilturk M, Tatar P, Kiraz N, Arpaç E, Sayılkan H (2008) Mater Res Bull 43:127–134
Zarrin S, Heshmatpour F (2018) J Hazard Mater 351:147–159
Ma J, Yang M, Sun Y, Li C, Li Q, Gao F, Yu F, Chen J (2014) Physica E 58:24–29
Liu Y, Zeng G, Tang L, Cai Y, Pang Y, Zhang Y, Yang G, Zhou Y, He X, He Y (2015) J Colloid Interface Sci 448:451–459
Zhao Y, Chen H, Li J, Chen C (2015) J Colloid Interface Sci 450:189–195
Sobhani-Nasab A, Eghbali-Arani M (2020) Seyed Mostafa Hosseinpour-Mashkani, Farhad Ahmadi, Mehdi Rahimi-Nasrabadi, Vahid Ameri. Iran J Catal 10:91–99
Funding
The authors are grateful to the council of Kosar University of Bojnord for providing financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was conducted following Compliance with Ethical Standards, and it did not involve human participants, animals, and potential conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sobhani, A., Alinavaz, S. Co-precipitation synthesis of CuMn2O4/CuMnO nanocomposites without capping agent and investigation of their applications for removing pollutants from wastewater. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-022-03732-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03732-2