Abstract
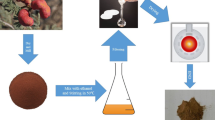
This study examined the corrosion inhibition of 304 stainless steel (304 SS) with fucoidan in 3.5% NaCl solution using both chemical and electrochemical methods, including mass loss as a chemical method and electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PP) as an electrochemical method. The fucoidan compound was verified and characterized by Fourier-transform infrared spectroscopy (FTIR), ultraviolet–visible spectroscopy, and morphology characteristics. The PP curves indicate that fucoidan was an effective corrosion inhibitor for 304 SS in 3.5% NaCl solutions, which indicates that the compound is a mixed-type inhibitor. It was shown that by adding the fucoidan inhibitor, the corrosion potential (Ecorr) and Tafel lines were slightly shifted. With the compound added, the value of the double-layer capacitance was reduced. In the case of 200 ppm, it reached maximum efficiencies of 81.7%. After studying its adsorption behavior on 304 SS, the Langmuir isotherm and chemical adsorption were concluded. It was necessary to compare the theoretical computations with the experimental findings using both density functional theory (DFT) and Monte Carlo simulations (MC).











Similar content being viewed by others
Data availability
The author confirms that the article’s data supporting this study are available.
References
Donik Č, Kocijan A (2014) Comparison of the corrosion behaviour of austenitic stainless steel in seawater and in a 35% NaCl solution. Materiali in tehnol 48(6):937–42
Palanisamy G (2019) Corrosion inhibitors. Corrosion inhibitors 24. https://doi.org/10.5772/intechopen.80542
Branzoi F, Branzoi V, Licu C (2014) Corrosion inhibition of carbon steel in cooling water systems by new organic polymers as green inhibitors. Mater Corros 65(6):637–647. https://doi.org/10.1002/maco.201206579
Marzorati S, Verotta L, Trasatti SP (2018) Green corrosion inhibitors from natural sources and biomass wastes. Molecules 24(1):48. https://doi.org/10.3390/molecules24010048
Argyropoulos V, Boyatzis SC, Giannoulaki M, Guilminot E, Zacharopoulou A (2021) Organic green corrosion inhibitors derived from natural and/or biological sources for conservation of metals cultural heritage. Microorganisms in the Deterioration and Preservation of Cultural Heritage 341. https://doi.org/10.1007/978-3-030-69411-1
Satapathy A, Gunasekaran G, Sahoo S, Amit K, Rodrigues P (2009) Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros Sci 51(12):2848–2856. https://doi.org/10.1016/j.corsci.2009.08.016
Hussin MH, Kassim MJ (2011) The corrosion inhibition and adsorption behavior of Uncaria gambir extract on mild steel in 1 M HCl. Mater Chem Phys 125(3):461. https://doi.org/10.1016/j.matchemphys.2010.10.032
Akalezi CO, Oguzie EE (2016) Evaluation of anticorrosion properties of Chrysophyllum albidum leaves extract for mild steel protection in acidic media. Intl J Ind Chem 7(1):81–92
Kumar KV, Pillai MSN, Thusnavis GR (2011) Seed extract of Psidium guajava as ecofriendly corrosion inhibitor for carbon steel in hydrochloric acid medium. J Mater Sci Technol 27(12):1143–1149. https://doi.org/10.1016/S1005-0302(12)60010-3
Berrissoul A, Ouarhach A, Benhiba F, Romane A, Zarrouk A, Guenbour A et al (2020) Evaluation of Lavandula mairei extract as green inhibitor for mild steel corrosion in 1 M HCl solution Experimental and theoretical approach. J Mol Liq 313:113493. https://doi.org/10.1016/j.molliq.2020.113493
Michael NC, Olubunmi JA (2014) The corrosion inhibition of mild steel in sulphuric acid solution by flavonoid (catechin) separated from Nypa fruticans Wurmb leaves extract. Sci J Chem 2(4):27–32. https://doi.org/10.11648/j.sjc.20140204.11
Sangeetha M, Rajendran S, Sathiyabama J, Krishnaveni A, Shanthy P, Manimaran N et al (2011) Corrosion inhibition by an aqueous extract of Phyllanthus amarus. Port Electrochim Acta 29(6):429–444. https://doi.org/10.4152/pea.201106429
Asadi N, Ramezanzadeh M, Bahlakeh G, Ramezanzadeh B (2019) Utilizing Lemon Balm extract as an effective green corrosion inhibitor for mild steel in 1M HCl solution: a detailed experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J Taiwan Inst Chem Eng 95:252–272. https://doi.org/10.1016/j.jtice.2018.07.011
Flores-De los Ríos J, Sánchez-Carrillo M, Nava-Dino C, Chacón-Nava J, González-Rodríguez J, Huape-Padilla E et al (2015) Opuntia ficus-indica extract as green corrosion inhibitor for carbon steel in 1 M HCl solution. J Spectrosc 12:1–9. https://doi.org/10.1155/2015/714692
Onen A, Buba J (2018) Ficus polita (Bush fig) leaves extract as corrosion inhibitor of aluminium in alkaline medium. J Chem Soc of Nigeria 43(1). https://www.journals.chemsociety.org.ng/index.php/jcsn/article/view/132
Wang J, Du M, Li G, Shi P (2022) Research progress on microbiological inhibition of corrosion: a review. Journal of Cleaner Production 133658https://doi.org/10.1016/j.jclepro.2022.133658
Calado R, Leal MC, Gaspar H, Santos S, Marques A, Nunes ML, et al (2018) How to succeed in marketing marine natural products for nutraceutical pharmaceutical and cosmeceutical markets. Grand challenges in marine biotechnology: Springer p. 317-403 https://doi.org/10.1007/978-3-319-69075-9_9
Wang C, Zhang J, Chen X-L, Xiang B, Duan J-Z, Hou B-R (2017) Inhibition of zinc corrosion by fucoidan in natural sea water. Acta Metallurgica Sinica (English Letters) 30(6):594–600. https://doi.org/10.1007/s40195-016-0524-9
Shabani-Nooshabadi M, Ghandchi M (2015) Santolina chamaecyparissus extract as a natural source inhibitor for 304 stainless steel corrosion in 3.5% NaCl. J Ind Eng Chem 31:231–237. https://doi.org/10.1016/j.jiec.2015.06.028
Fouda A, Wahba A, Bonayan AM (2021) Corrosion inhibition of stainless steel in 10 M hydrochloric acid solution using novel nonionic surfactant: electrochemical and density functional theory/B3LYP/6–31G* analysis. Surf Eng Appl Electrochem 57(6):689–702. https://doi.org/10.3103/S1068375521060041
Martinelli-Orlando F, Angst U (2022) Monitoring corrosion rates with ER-probes—a critical assessment based on experiments and numerical modelling. Corros Eng, Sci Technol 57(3):254–268. https://doi.org/10.1080/1478422X.2022.2053036
Shen Q, Zhang Y, Li X, Wang L, Nie C (2022) Enhanced wear resistance and corrosion resistance of 304 austenitic stainless steel by duplex surface treatment. Steel Res Intl 2100689. https://doi.org/10.1002/srin.202100689
MacKay JR, Smith MJ, van Keulen F, Bosman TN, Pegg NG (2010) Experimental investigation of the strength and stability of submarine pressure hulls with and without artificial corrosion damage. Mar Struct 23(3):339–359. https://doi.org/10.1016/j.marstruc.2010.06.001
Lemallem SE, Fiala A, Ladouani HB, Allal H (2022) Corrosion inhibition performance of two ketene dithioacetal derivatives for stainless steel in hydrochloric acid solution. J Electrochem Sci Technol 13(2):237–53. https://doi.org/10.33961/jecst.2021.00822
Pockett A, Spence M, Thomas SK, Raptis D, Watson T, Carnie MJ (2021) Beyond the first quadrant: origin of the high frequency intensity-modulated photocurrent/photovoltage spectroscopy response of perovskite solar cells. Solar RRL 5(5):2100159. https://doi.org/10.1002/solr.202100159
Mameri S, Boughrara D, Lazar F, Chopart J-P (2022) Effects of cathodic polarization on the corrosion behavior of X60 pipeline steel in a simulated soil solution. Steel Res Int 220005. https://doi.org/10.1002/srin.202200050
Hamilton-Amachree A, Iroha NB (2020) Corrosion inhibition of API 5L X80 pipeline steel in acidic environment using aqueous extract of Thevetia peruviana. Chem Int 6(3):110. https://doi.org/10.5281/zenodo.3516565
Iroha NB, Maduelosi NJ (2021) Corrosion inhibitive action and adsorption behaviour of justicia secunda leaves extract as an eco-friendly inhibitor for aluminium in acidic media. Biointerface Res Appl Chem 11:13019–30. https://doi.org/10.33263/BRIAC115.1301913030
Ahmed L, Rebaz O (2021) 1H-pyrrole, furan, and thiophene molecule corrosion inhibitor behaviors. J Phys Chem Funct Mater 4(2):1–4. https://doi.org/10.54565/jphcfum.989851
Roy S, Zhang X, Puthirath AB, Meiyazhagan A, Bhattacharyya S, Rahman MM et al (2021) Structure, properties and applications of two-dimensional hexagonal boron nitride. Adv Mater 33(44):2101589. https://doi.org/10.1002/adma.202101589
Kasprzhitskii A, Lazorenko G (2021) Corrosion inhibition properties of small peptides: DFT and Monte Carlo simulation studies. J Mol Liq 331:115782. https://doi.org/10.1016/j.molliq.2021.115782
Rajasekar P, Palanisamy S, Anjali R, Vinosha M, Elakkiya M, Marudhupandi T et al (2019) Isolation and structural characterization of sulfated polysaccharide from Spirulina platensis and its bioactive potential: in vitro antioxidant, antibacterial activity and Zebrafish growth and reproductive performance. Int J Biol Macromol 141:809–821. https://doi.org/10.1016/j.ijbiomac.2019.09.024
Manikandan R, Parimalanandhini D, Mahalakshmi K, Beulaja M, Arumugam M, Janarthanan S et al (2020) Studies on isolation, characterization of fucoidan from brown algae Turbinaria decurrens and evaluation of its in vivo and in vitro anti-inflammatory activities. Int J Biol Macromol 160:1263–1276. https://doi.org/10.1016/j.ijbiomac.2020.05.152
Koh HSA, Lu J, Zhou W (2019) Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohyd Polym 212:178–185. https://doi.org/10.1016/j.carbpol.2019.02.040
Al Monla R, Dassouki Z, Sari-Chmayssem N, Mawlawi H, Gali-Muhtasib H (2022) Fucoidan and alginate from the brown algae Colpomenia sinuosa and their combination with vitamin C trigger apoptosis in colon cancer. Molecules 27(2):358. https://doi.org/10.3390/molecules27020358
Li W, Zhang Z, Zhai Y, Ruan L, Zhang W, Wu L (2020) Electrochemical and computational studies of proline and captopril as corrosion inhibitors on carbon steel in a phase change material solution. Int J Electrochem Sci 15(1):722–739. https://doi.org/10.20964/2020.01.63
Ren X, Xu S, Gu X, Tan B, Hao J, Feng L et al (2021) Hyperbranched molecules having multiple functional groups as effective corrosion inhibitors for Al alloys in aqueous NaCl. J Colloid Interface Sci 585:614–626. https://doi.org/10.1016/j.jcis.2020.10.041
Abdulazeez I, Zeino A, Kee CW, Al-Saadi AA, Khaled M, Wong MW et al (2019) Mechanistic studies of the influence of halogen substituents on the corrosion inhibitive efficiency of selected imidazole molecules: a synergistic computational and experimental approach. Appl Surf Sci 471:494–505. https://doi.org/10.1016/j.apsusc.2018.12.028
Rezaeivala M, Karimi S, Sayin K, Tüzün B (2022) Experimental and theoretical investigation of corrosion inhibition effect of two piperazine-based ligands on carbon steel in acidic media. Colloids Surf, A 641:128538. https://doi.org/10.1016/j.colsurfa.2022.128538
Fouda AE-AS, El-Askalany AH, Molouk AF, Elsheikh NS, Abousalem AS (2021) Experimental and computational chemical studies on the corrosion inhibitive properties of carbonitrile compounds for carbon steel in aqueous solutions. Sci Rep 11(1):1–24
Al-Amiery AA, Mohamad AB, Kadhum AAH, Shaker LM, Isahak WNRW, Takriff MS (2022) Experimental and theoretical study on the corrosion inhibition of mild steel by nonanedioic acid derivative in hydrochloric acid solution. Sci Rep 12(1):1–21. https://doi.org/10.1038/s41598-022-08146-8
Hamee RSA, Aljohani MM, Essa AB, Khaled A, Amr M, Nassar MM, Badr SR, Al-Mhyawi MS, Soliman, (2021) Electrochemical techniques for evaluation of expired megavit drugs as corrosion inhibitor for steel in hydrochloric acid. Int J Electrochem Sci 16:1–13. https://doi.org/10.20964/2021.04.15
Mobin M, Parveen M, Aslam R (2022) Effect of different additives, temperature, and immersion time on the inhibition behavior of L-valine for mild steel corrosion in 5% HCl solution. J Phys Chem Solids 161:110422. https://doi.org/10.1016/j.jpcs.2021.110422
Hsissou R, Benhiba F, Dagdag O, El Bouchti M, Nouneh K, Assouag M et al (2020) Development and potential performance of prepolymer in corrosion inhibition for carbon steel in 1.0 M HCl: outlooks from experimental and computational investigations. J Colloid Interface Sci 574:43–60. https://doi.org/10.1016/j.jcis.2020.04.022
Darweesh MA, Elgendy MY, Ayad MI, Ahmed AM, Elsayed NK, Hammad W (2022) Adsorption isotherm, kinetic, and optimization studies for copper (II) removal from aqueous solutions by banana leaves and derived activated carbon. S Afr J Chem Eng 40:10–20. https://doi.org/10.1016/j.sajce.2022.01.002
Verma C, Verma DK, Ebenso EE, Quraishi MA (2018) Sulfur and phosphorus heteroatom-containing compounds as corrosion inhibitors: an overview. Heteroat Chem 29(4):e21437. https://doi.org/10.1002/hc.21437
Abd E-AFS, Ali AH (2018) Egy-dronate drug as promising corrosion inhibitor of C-steel in aqueous medium. Zaštita materijala 59(1):126–40. https://scindeks.ceon.rs/article.aspx?artid=0351-94651801126A
Qiang Y, Guo L, Li H, Lan X (2021) Fabrication of environmentally friendly Losartan potassium film for corrosion inhibition of mild steel in HCl medium. Chem Eng J 406:126863. https://doi.org/10.1016/j.cej.2020.126863
Nsude OP, Orie KJ (2022) Thermodynamic and adsorption analysis of corrosion inhibition of mild steel in 0.5 M HCl medium via ethanol extracts of Phyllanthus mellerianus. Am J Appl Chem 10(3):67–75. https://doi.org/10.11648/j.ajac.20221003.12
Sasikala T, Priya KG, Akila A (2022) An investigation on corrosion inhibition efficacy of benzodiazepines on mild steel in acid medium. Mater Today: Proc 49:2205–2211. https://doi.org/10.1016/j.matpr.2021.09.318
Okoli CP, Guo QJ, Adewuyi GO (2014) Application of quantum descriptors for predicting adsorption performance of starch and cyclodextrin adsorbents. Carbohyd Polym 101:40–49. https://doi.org/10.1016/j.carbpol.2013.08.065
Fouda A, Etaiw S, El-Habab AT, Wahba A (2020) Synthesis, characterization, and application of new nonionic surfactant as a corrosion inhibitor for carbon steel in 1 M hydrochloric acid solution. Jo Bio-and Tribo-Corrosion 6(3):1–9. https://doi.org/10.1007/s40735-020-00373-8
Assad H, Ganjoo R, Sharma S, editors (2022) A theoretical insight to understand the structures and dynamics of thiazole derivatives. Journal of Physics: Conference Series;: IOP Publishing. https://doi.org/10.1088/1742-6596/2267/1/012063
Ouakki M, Galai M, Rbaa M, Abousalem AS, Lakhrissi B, Touhami ME et al (2020) Electrochemical, thermodynamic and theoretical studies of some imidazole derivatives compounds as acid corrosion inhibitors for mild steel. J Mol Liq 319:114063. https://doi.org/10.1016/j.molliq.2020.114063
Verma C, Lgaz H, Verma D, Ebenso EE, Bahadur I, Quraishi M (2018) Molecular dynamics and Monte Carlo simulations as powerful tools for study of interfacial adsorption behavior of corrosion inhibitors in aqueous phase: a review. J Mol Liq 260:99–120. https://doi.org/10.1016/j.molliq.2018.03.045
Madkour LH, Kaya S, Obot IB (2018) Computational, Monte Carlo simulation and experimental studies of some arylazotriazoles (AATR) and their copper complexes in corrosion inhibition process. J Mol Liq 260:351–374. https://doi.org/10.1016/j.molliq.2018.01.055
Tantawy AH, Soliman KA, Abd El-Lateef HM (2020) Novel synthesized cationic surfactants based on natural piper nigrum as sustainable-green inhibitors for steel pipeline corrosion in CO2–35% NaCl: DFT Monte Carlo simulations and experimental approaches. J Clean Prod 250:119510
Xu X, Liu S, Liu Y, Smith K, Cui Y (2019) Corrosion of stainless steel valves in a reverse osmosis system: analysis of corrosion products and metal loss. Eng Fail Anal 105:40–51. https://doi.org/10.1016/j.engfailanal.2019.06.026
Pareek S, Jain D, Hussain S, Biswas A, Shrivastava R, Parida SK et al (2019) A new insight into corrosion inhibition mechanism of copper in aerated 3.5 wt.% NaCl solution by eco-friendly imidazopyrimidine dye: experimental and theoretical approach. Chem Eng J 358:725–742. https://doi.org/10.1016/j.cej.2018.08.079
Zhou J, Niu X, Cui Y, Wang Z, Wang J, Wang R (2020) Study on the film forming mechanism, corrosion inhibition effect and synergistic action of two different inhibitors on copper surface chemical mechanical polishing for GLSI. Appl Surf Sci 505:144507. https://doi.org/10.1016/j.apsusc.2019.144507
Acknowledgements
The authors would like to extend their appreciation to the Deanship of Scientific Research at the University of Tabuk for funding this work through research group number S-1442-0108.
Funding
The Deanship of Scientific Research at the University of Tabuk through research group number S-1442–0108.
Author information
Authors and Affiliations
Contributions
This work has been done by all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keshk, A.A., Elsayed, N.H., Almutairi, F.M. et al. Effect of green and sustainable extracted fucoidan polysaccharide as a corrosion inhibitor in 3.5% NaCl. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-022-03579-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03579-7