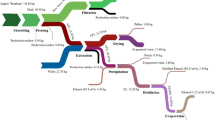
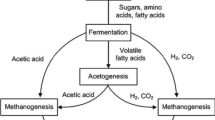
Abstract
Apples are among the most consumed fruits worldwide, being consumed as fresh fruit or either processed into juice, vinegar, cider, and others. The by-products generated by apple processing industry are usually discarded without further application. This study addressed the valorization of apple pomace using dry anaerobic digestion technology in semi-continuous mode. The operational parameters, volatile fatty acids, microbial community, and biogas production were evaluated during 40 days of digestion. The potential for bioenergy production, avoided greenhouse gas emissions, and the energy balance were calculated to determine the possibilities for implementing an anaerobic biorefinery in the apple processing industry. The results showed a microbial community of the reactor composed of bacteria (97.5%) and Archaea (2.5%), where Amphibacillus was the dominant genus. The methane yield obtained was 36.61 L CH4 kg−1 TVSremoved, which can generate 1.92 kWh ton−1 of electricity and 8.63 MJ ton−1 of heat, avoiding 0.62 kg CO2-eq ton−1 apple pomace submitted to anaerobic digestion. The bioenergy recovered could supply 19.18% electricity and 11.15% heat of the anaerobic reactor used in the designed anaerobic biorefinery process. In conclusion, anaerobic digestion can be a promising approach for the management of apple pomace, decreasing greenhouse gas emissions and contributing to the circular economy transition of the apple processing industry.
Graphical Abstract






Similar content being viewed by others
Data availability
Data available on request.
Abbreviations
- AD:
-
Anaerobic digestion
- AP:
-
Apple pomace
- AVS:
-
Amplicon sequence variant
- CF:
-
Conversion factor from MJ to MWh
- CHP:
-
Combined heat and power
- C m :
-
Percentage of methane in biogas
- COD:
-
Chemical oxygen demand
- DADA:
-
Divisive amplicon denoising algorithm
- \(EF_{CO_2-EG}\) :
-
Emission factor of electric energy
- \(EF_{CO_2-HG}\) :
-
Emission factor of heat energy
- \(EG_{CH_4}\) :
-
Electricity generation
- EMY added :
-
Experimental methane yield based on volatile solids added
- EMY removed :
-
Experimental methane yield based on volatile solids removed
- FID:
-
Flame ionization detector
- GC:
-
Gas chromatograph
- GHG:
-
Greenhouse gas
- GHG electricity :
-
Avoided greenhouse gas emissions from electricity
- GHG heat :
-
Avoided greenhouse gas emissions from heat
- \(HG_{CH_4}\) :
-
Heat generated
- HRT:
-
Hydraulic retention time
- \(LCV_{CH_4}\) :
-
Lower calorific value of methane
- NCBI:
-
National Center for Biotechnology Information
- η e :
-
Engine efficiency for electricity
- η t :
-
Engine efficiency for thermal energy
- OLR:
-
Organic loading rate
- PCR:
-
Polymerase chain reaction
- Q biogas :
-
Biogas volume
- Q feed :
-
Flow of feed
- QIIME:
-
Quantitative insights into microbial ecology
- S feed :
-
Concentration of COD or TVS in the feed
- VFA:
-
Volatile fatty acids
- VSR:
-
Volatile solids loading rate
- UASB:
-
Upflow anaerobic sludge blanket
- TCD:
-
Thermal conductivity detector
- TFS:
-
Total fixed solids
- TS:
-
Total solids
- TVS:
-
Total volatile solids
References
Lyu F, Luiz SF, Azeredo DRP, et al (2020) Apple pomace as a functional and healthy ingredient in food products: a review. Processes 8
Zhang F, Wang T, Wang X, Lü X (2021) Apple pomace as a potential valuable resource for full-components utilization: a review. J Clean Prod 329:129676. https://doi.org/10.1016/j.jclepro.2021.129676
Delphi L, Sepehri H (2016) Apple pectin: a natural source for cancer suppression in 4T1 breast cancer cells in vitro and express p53 in mouse bearing 4T1 cancer tumors, in vivo. Biomed Pharmacother 84:637–644. https://doi.org/10.1016/j.biopha.2016.09.080
Antonic B, Jancikova S, Dordevic D, Tremlova B (2020) Apple pomace as food fortification ingredient: a systematic review and meta-analysis. J Food Sci 85:2977–2985. https://doi.org/10.1111/1750-3841.15449
FAOSTAT F and AO of the UN (2020) FAOSTAT statistical database. In: Food Agric. Organ. United Nations. https://www.fao.org/faostat/en/#data
Lazzarotto JJ, Girardi CL, Zandoná GP (2016) Parâmetros para investimentos na produção de suco integral de maçã com alto padrão tecnológico. Bento Gonçalves
Magyar M, da Costa SL, Jin M et al (2016) Conversion of apple pomace waste to ethanol at industrial relevant conditions. Appl Microbiol Biotechnol 100:7349–7358. https://doi.org/10.1007/s00253-016-7665-7
Wang X, Kristo E, LaPointe G (2019) The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll 91. https://doi.org/10.1016/j.foodhyd.2019.01.004
Paini J, Benedetti V, Menin L et al (2021) Subcritical water hydrolysis coupled with hydrothermal carbonization for apple pomace integrated cascade valorization. Bioresour Technol 342:125956. https://doi.org/10.1016/j.biortech.2021.125956
Gullón B, Falqué E, Alonso JL, Parajó JC (2007) Evaluation of apple pomace as a raw material for alternative applications in food industries. Food Technol Biotechnol 45:426–433
Arraibi AA, Liberal Â, Dias MI et al (2021) Chemical and bioactive characterization of Spanish and Belgian apple pomace for its potential use as a novel dermocosmetic formulation. Foods 10:1–14. https://doi.org/10.3390/foods10081949
Luo J, Xu Y (2020) Comparison of biological and chemical pretreatment on coproduction of pectin and fermentable sugars from apple pomace. Appl Biochem Biotechnol 190:129–137. https://doi.org/10.1007/s12010-019-03088-w
Sadhukhan J, Martinez-Hernandez E, Amezcua-Allieri MA et al (2019) Economic and environmental impact evaluation of various biomass feedstock for bioethanol production and correlations to lignocellulosic composition. Bioresour Technol Reports 7:100230. https://doi.org/10.1016/j.biteb.2019.100230
de Oliveira TCG, Sganzerla WG, Ampese LC et al (2022) Sustainable valorization of apple waste in a biorefinery: a bibliometric analysis. Biofuels, Bioprod Biorefining 16:891–919. https://doi.org/10.1002/bbb.2343
Wadhwa M, Bakshi MPS, Makkar HPS (2015) Wastes to worth: value added products from fruit and vegetable wastes. CAB Int 43:1–25
Ampese LC, Ziero HDD, Velásquez J et al (2022) Apple pomace management by anaerobic digestion and composting: a life cycle assessment. Biofuels, Bioprod Biorefining n/a. https://doi.org/10.1002/bbb.2446
Sawatdeenarunat C, Surendra KC, Takara D, et al (2015) Anaerobic digestion of lignocellulosic biomass: challenges and opportunities. Bioresour. Technol. 178
Atasoy M, Owusu-Agyeman I, Plaza E, Cetecioglu Z (2018) Bio-based volatile fatty acid production and recovery from waste streams: current status and future challenges. Bioresour Technol 268:773–786. https://doi.org/10.1016/j.biortech.2018.07.042
Braune M, Yuan B, Sträuber H, et al (2021) A downstream processing cascade for separation of caproic and caprylic acid from maize silage-based fermentation broth. Front. Bioeng. Biotechnol. 9
Cheah WY, Sankaran R, Show PL et al (2020) Pretreatment methods for lignocellulosic biofuels production: current advances, challenges and future prospects. Biofuel Res J 7:1115–1127. https://doi.org/10.18331/BRJ2020.7.1.4
Sun Q, Li H, Yan J et al (2015) Selection of appropriate biogas upgrading technology-a review of biogas cleaning, upgrading and utilisation. Renew Sustain Energy Rev 51:521–532. https://doi.org/10.1016/j.rser.2015.06.029
Ricciardi P, Cillari G, CarnevaleMiino M, Collivignarelli MC (2020) Valorization of agro-industry residues in the building and environmental sector: a review. Waste Manag Res 38:487–513. https://doi.org/10.1177/0734242X20904426
dos Santos LA, Valença RB, da Silva LCS et al (2020) Methane generation potential through anaerobic digestion of fruit waste. J Clean Prod 256:120389. https://doi.org/10.1016/j.jclepro.2020.120389
Kothari R, Pandey AK, Kumar S et al (2014) Different aspects of dry anaerobic digestion for bio-energy: an overview. Renew Sustain Energy Rev 39:174–195. https://doi.org/10.1016/j.rser.2014.07.011
Wainaina S, Lukitawesa, Kumar Awasthi M, Taherzadeh MJ (2019) Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: a critical review. Bioengineered 10:437–458. https://doi.org/10.1080/21655979.2019.1673937
Weber CT, Trierweiler LF, Trierweiler JO (2020) Food waste biorefinery advocating circular economy: bioethanol and distilled beverage from sweet potato. J Clean Prod 268:121788. https://doi.org/10.1016/j.jclepro.2020.121788
Keijer T, Bakker V, Slootweg JC (2019) Circular chemistry to enable a circular economy. Nat Chem 11:190–195. https://doi.org/10.1038/s41557-019-0226-9
Mao C, Feng Y, Wang X, Ren G (2015) Review on research achievements of biogas from anaerobic digestion. Renew Sustain Energy Rev 45:540–555. https://doi.org/10.1016/j.rser.2015.02.032
Khan MA, Ngo HH, Guo WS et al (2016) Optimization of process parameters for production of volatile fatty acid, biohydrogen and methane from anaerobic digestion. Bioresour Technol 219:738–748. https://doi.org/10.1016/j.biortech.2016.08.073
Green MR, Sambrook J (2012) Molecular cloning. A Lab Man 4th
Andrews S, Krueger F, Segonds-Pichon A, et al (2010) FastQC. A Qual Control tool high throughput Seq data 370:
Bolyen E, Rideout JR, Dillon MR et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
APHA APHA (2017) Standard methods for the examination of water and wastewater, 22nd ed
Campello LD, Barros RM, Tiago Filho GL, dos Santos IFS (2021) Analysis of the economic viability of the use of biogas produced in wastewater treatment plants to generate electrical energy. Environ Dev Sustain 23:2614–2629. https://doi.org/10.1007/s10668-020-00689-y
dos Santos MA, Damázio JM, Rogério JP et al (2017) Estimates of GHG emissions by hydroelectric reservoirs: the Brazilian case. Energy 133:99–107. https://doi.org/10.1016/j.energy.2017.05.082
MCTIC, Ministério da Ciência T e I (2021) Fator médio-inventários corporativos. In: Sist. Interligado Nac. do Bras.
IPCC NGGIP (2006) 2006 IPCC guidelines for national greenhouse gas inventories. Hayama
Melikoğlu AY, Bilek SE, Cesur S (2019) Optimum alkaline treatment parameters for the extraction of cellulose and production of cellulose nanocrystals from apple pomace. Carbohydr Polym 215:330–337. https://doi.org/10.1016/j.carbpol.2019.03.103
Meegoda JN, Li B, Patel K, Wang LB (2018) A review of the processes, parameters, and optimization of anaerobic digestion. Public Heal, Int. J. Environ. Res, p 15
Xing T, Wang Z, Zhen F et al (2022) Initial pH-driven production of volatile fatty acid from hybrid Pennisetum. Bioresour Technol 347:126426. https://doi.org/10.1016/j.biortech.2021.126426
Guerrero MRB, da Silva M, Paula M, Zaragoza MM et al (2014) Thermogravimetric study on the pyrolysis kinetics of apple pomace as waste biomass. Int J Hydrogen Energy 39:16619–16627. https://doi.org/10.1016/j.ijhydene.2014.06.012
Pathania S, Sharma N, Handa S (2017) Immobilization of co-culture of Saccharomyces cerevisiae and Scheffersomyces stipitis in sodium alginate for bioethanol production using hydrolysate of apple pomace under separate hydrolysis and fermentation. Biocatal Biotransformation 35:450–459. https://doi.org/10.1080/10242422.2017.1368497
Leonel LV, Sene L, da Cunha MAA et al (2020) Valorization of apple pomace using bio-based technology for the production of xylitol and 2G ethanol. Bioprocess Biosyst Eng 43:2153–2163. https://doi.org/10.1007/s00449-020-02401-w
Aino K, Hirota K, Okamoto T, et al (2018) Microbial communities associated with indigo fermentation that thrive in anaerobic alkaline environments. Front. Microbiol. 9
Yang C, Niu Y, Su H et al (2010) A novel microbial habitat of alkaline black liquor with very high pollution load: microbial diversity and the key members in application potentials. Bioresour Technol 101:1737–1744. https://doi.org/10.1016/j.biortech.2009.09.092
Li X, Liu G, Liu S et al (2018) The relationship between volatile fatty acids accumulation and microbial community succession triggered by excess sludge alkaline fermentation. J Environ Manage 223:85–91. https://doi.org/10.1016/j.jenvman.2018.06.002
Zhilina TN, Garnova ES, Tourova TP et al (2001) Amphibacillus fermentum sp. nov. and Amphibacillus tropicussp. nov., new alkaliphilic, facultatively anaerobic. Saccharolytic bacilli from Lake Magadi Microbiol 70:711–722. https://doi.org/10.1023/A:1013196017556
Lin L, Wen L, Chen S et al (2015) Effect of alkaline treatment pattern on anaerobic fermentation of swine manure. Process Biochem 50:1710–1717. https://doi.org/10.1016/j.procbio.2015.08.011
Pugin B, Blamey JM, Baxter BK, Wiegel J (2012) Amphibacillus cookii sp. nov., a facultatively aerobic, spore-forming, moderately halophilic, alkalithermotolerant bacterium. Int J Syst Evol Microbiol 62:2090–2096. https://doi.org/10.1099/ijs.0.034629-0
Xiang Q, Yang K, Chen Z, et al (2022) A novel and green method for turning food waste into environmentally-friendly organic deicing salts: enhanced VFA production through AnMBR. Sep. 9
Anderson K, Sallis P, Uyanik S (2003) 24—Anaerobic treatment processes. In: Mara D, Horan NBT-H of W and WM (eds). Academic Press, London, pp 391–426
Liu X, Yan Z, Yue Z-B (2011) 3.10—Biogas. In: Moo-Young MBT-CB (Second E (ed). Academic Press, Burlington, pp 99–114
Wang C, Li Y, Sun Y (2020) Acclimation of acid-tolerant methanogenic culture for bioaugmentation: strategy comparison and microbiome succession. ACS Omega 5:6062–6068. https://doi.org/10.1021/acsomega.9b03783
Brenner DJ, Krieg NR, Staley JT, Garrity GM (2005) Bergey’s Manual® of systematic bacteriology, 2nd edn. Springer, New York, NY
Carballa M, Regueiro L, Lema JM (2015) Microbial management of anaerobic digestion: exploiting the microbiome-functionality nexus. Curr Opin Biotechnol 33:103–111. https://doi.org/10.1016/j.copbio.2015.01.008
Baldwin SA, Khoshnoodi M, Rezadehbashi M et al (2015) The microbial community of a passive biochemical reactor treating arsenic, zinc, and sulfate-rich seepage. Front Bioeng Biotechnol 3:1–13. https://doi.org/10.3389/fbioe.2015.00027
Imachi H, Sakai S (2016) Methanolinea. Bergey’s Man. Syst. Archaea Bact. 1–4
Imachi H, Sakai S, Sekiguchi Y et al (2008) Methanolinea tarda gen. nov., sp. nov. a methane-producing archaeon isolated from a methanogenic digester sludge. Int J Syst Evol Microbiol 58:294–301. https://doi.org/10.1099/ijs.0.65394-0
Raposo F, De la Rubia MA, Fernández-Cegrí V, Borja R (2012) Anaerobic digestion of solid organic substrates in batch mode: an overview relating to methane yields and experimental procedures. Renew Sustain Energy Rev 16:861–877. https://doi.org/10.1016/j.rser.2011.09.008
Angelidaki I, Sanders W (2004) Assessment of the anaerobic biodegradability of macropollutants. Re/Views Environ Sci Bio/Technology 3:117–129. https://doi.org/10.1007/s11157-004-2502-3
Jain S, Jain S, Wolf IT et al (2015) A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew Sustain Energy Rev 52:142–154. https://doi.org/10.1016/j.rser.2015.07.091
Jewell WJ, Cummings RJ (1984) Apple pomace energy and solids recovery. J Food Sci 49:407–410. https://doi.org/10.1111/j.1365-2621.1984.tb12433.x
Aceves-Lara C-A, Latrille E, Conte T, Steyer J-P (2012) Online estimation of VFA, alkalinity and bicarbonate concentrations by electrical conductivity measurement during anaerobic fermentation. Water Sci Technol 65:1281–1289. https://doi.org/10.2166/wst.2012.703
Laiq Ur Rehman M, Iqbal A, Chang C-C et al (2019) Anaerobic digestion. Water Environ Res 91:1253–1271. https://doi.org/10.1002/wer.1219
Yang L, Huang Y, Zhao M et al (2015) Enhancing biogas generation performance from food wastes by high-solids thermophilic anaerobic digestion: effect of pH adjustment. Int Biodeterior Biodegradation 105:153–159. https://doi.org/10.1016/j.ibiod.2015.09.005
Duong TH, Grolle K, Nga TTV et al (2019) Protein hydrolysis and fermentation under methanogenic and acidifying conditions. Biotechnol Biofuels 12:254. https://doi.org/10.1186/s13068-019-1592-7
Chen X, Yan W, Sheng K, Sanati M (2014) Comparison of high-solids to liquid anaerobic co-digestion of food waste and green waste. Bioresour Technol 154:215–221. https://doi.org/10.1016/j.biortech.2013.12.054
Adou KE, Alle OA, Kouakou AR et al (2020) Anaerobic mono-digestion of wastewater from the main slaughterhouse in Yamoussoukro (Côte d’Ivoire): evaluation of biogas potential and removal of organic pollution. J Environ Chem Eng 8:103770. https://doi.org/10.1016/j.jece.2020.103770
Hu Z, Grasso D (2005) Water analysis | chemical oxygen demand. In: Second E (ed) Worsfold P, Townshend A, Poole CBT-E of AS. Elsevier, Oxford, pp 325–330
Atelge MR, Atabani AE, Banu JR et al (2020) A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 270:117494. https://doi.org/10.1016/j.fuel.2020.117494
Lohani SP, Havukainen J (2018) Anaerobic digestion: factors affecting anaerobic digestion process BT—waste bioremediation. In: Gnansounou E, Gurunathan B et al (eds) Varjani SJ. Springer Singapore, Singapore, pp 343–359
Edwiges T, Bastos JA, Lima Alino JH et al (2019) Comparison of various pretreatment techniques to enhance biodegradability of lignocellulosic biomass for methane production. J Environ Chem Eng 7:103495. https://doi.org/10.1016/j.jece.2019.103495
Wang W, Lee D-J (2021) Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: a review. Bioresour Technol 339:125587. https://doi.org/10.1016/j.biortech.2021.125587
Zhang C, Su H, Baeyens J, Tan T (2014) Reviewing the anaerobic digestion of food waste for biogas production. Renew Sustain Energy Rev 38:383–392. https://doi.org/10.1016/j.rser.2014.05.038
Kainthola J, Kalamdhad AS, Goud VV (2019) A review on enhanced biogas production from anaerobic digestion of lignocellulosic biomass by different enhancement techniques. Process Biochem 84:81–90. https://doi.org/10.1016/j.procbio.2019.05.023
Wu D, Peng X, Li L et al (2021) Commercial biogas plants: review on operational parameters and guide for performance optimization. Fuel 303:121282. https://doi.org/10.1016/j.fuel.2021.121282
Wagner AO, Lackner N, Mutschlechner M, et al (2018) Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production. Energies 11
Khan MU, Ahring BK (2019) Lignin degradation under anaerobic digestion: influence of lignin modifications—a review. Biomass Bioenerg 128:105325. https://doi.org/10.1016/j.biombioe.2019.105325
Arenas-Cárdenas P, López-López A, Moeller-Chávez GE, León-Becerril E (2017) Current pretreatments of lignocellulosic residues in the production of bioethanol. Waste Biomass Valorization 8:161–181. https://doi.org/10.1007/s12649-016-9559-4
Kumari D, Singh R (2018) Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renew Sustain Energy Rev 90:877–891. https://doi.org/10.1016/j.rser.2018.03.111
Muharja M, Junianti F, Ranggina D et al (2018) An integrated green process: subcritical water, enzymatic hydrolysis, and fermentation, for biohydrogen production from coconut husk. Bioresour Technol 249:268–275. https://doi.org/10.1016/j.biortech.2017.10.024
Claes A, Melchi L, Uludag-Demirer S, Demirer GN (2021) Supplementation of carbon-based conductive materials and trace metals to improve biogas production from apple pomace. Sustainability 13
Molinuevo-Salces B, Riaño B, Hijosa-Valsero M et al (2020) Valorization of apple pomaces for biofuel production: a biorefinery approach. Biomass Bioenerg 142:105785. https://doi.org/10.1016/j.biombioe.2020.105785
Dubrovskis V, Plume I (2017) Biogas from wastes of pumpkin, marrow and apple. Agron Res 15:69–78
Olabi AG, Abdelkareem MA (2022) Renewable energy and climate change. Renew Sustain Energy Rev 158:112111. https://doi.org/10.1016/j.rser.2022.112111
Obileke K, Onyeaka H, Omoregbe O et al (2022) Bioenergy from bio-waste: a bibliometric analysis of the trend in scientific research from 1998–2018. Biomass Convers Biorefinery 12:1077–1092. https://doi.org/10.1007/s13399-020-00832-9
Cheng J, Wang Q, Yu J (2022) Life cycle assessment of concentrated apple juice production in China: mitigation options to reduce the environmental burden. Sustain Prod Consum 32:15–26. https://doi.org/10.1016/j.spc.2022.04.006
Bernstad A, la Cour JJ (2011) A life cycle approach to the management of household food waste—a Swedish full-scale case study. Waste Manag 31:1879–1896. https://doi.org/10.1016/j.wasman.2011.02.026
Khanh Nguyen V, Kumar Chaudhary D, Hari Dahal R et al (2021) Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 285:119105. https://doi.org/10.1016/j.fuel.2020.119105
(2022) Silva. In: high qual. ribosomal RNA databases. https://www.arb-silva.de. Accessed 30 May 2022
NCBI (2022) National Center for Biotechnology Information. In: Database. https://www.ncbi.nlm.nih.gov. Accessed 30 May 2022
Funding
This work was supported by the Brazilian Science and Research Foundation (CNPq, Brazil) (productivity grants 302451/2021–8); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) (Finance code 001); and São Paulo Research Foundation (FAPESP, Brazil) (grant numbers 2018/14938–4 for T.F.C.; 2019/26925–7 for W.G.S.; 2021/03950–6 for J.M.C.).
Author information
Authors and Affiliations
Contributions
LCA: conceptualization; data curation; writing — original draft; review and editing. WGS: data curation; writing — original draft; review and editing. HDDZ: data curation; writing — original draft; review and editing. JMC: data curation; writing — original draft; review and editing. GM: writing — review of original draft and editing; supervision. TF-C: writing — review of original draft and editing; supervision; funding acquisition.
Corresponding authors
Ethics declarations
Ethical approval
None.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ampese, L.C., Sganzerla, W.G., Di Domenico Ziero, H. et al. Valorization of apple pomace for biogas production: a leading anaerobic biorefinery approach for a circular bioeconomy. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03534-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03534-6