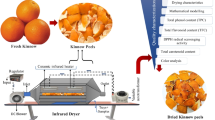
Abstract
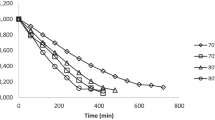
In this study, the effect of different infrared (IR) drying temperatures (60, 80, 100, and 120 °C) on drying kinetics and quality attributes of shea fruit peel and pulp was investigated.The results showed that drying time decreased with an increase in temperature, with shorter drying time required for the peel at the same temperature than the pulp. The Logarithmic and Page models were the best in describing the drying process of the shea fruit peel and pulp, respectively. Moisture diffusivity increased from 1.01 × 10−9 to 3.56 × 10−9 m2/s and 1.10 × 10−9 to 4.75 × 10−9 m2/s for the peel and pulp, respectively, as the drying temperature increased from 60 to 120 °C. The activation energy was 23.47 kJ/mol for the peel and 27.19 kJ/mol for the pulp. The rehydration ratio (RR) and CIE-L*a*b* color values were influenced (p < 0.05) by the drying temperature with lower total color change (∆E) values observed at 60 °C and 80 °C. However, an increase in IR drying temperature did not significantly (p > 0.05) influence the calcium and magnesium contents of the dried shea fruit components. Drying of the peel and pulp above 80 °C reduced ß-carotene content, total phenol content (TPC), and total antioxidant activity (TAA) while the highest potassium, vitamin C, and total flavonoid content (TFC) retention were observed at 120 °C. The study suggests IR drying of shea fruit peel and pulp at 60 to 80 °C for efficient drying and maintenance of color, ß-carotene content, TPC, TAA, and higher RR. Where, fast drying, vitamin C and TFC are of interest, drying of shea fruit components at 120 °C is recommended.






Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this manuscript.
References
Naughton CC, Lovett PN, Mihelcic JR (2015) Land suitability modeling of shea (Vitellaria paradoxa) distribution across sub-Saharan Africa. Appl Geogr 58:217–227. https://doi.org/10.1016/j.apgeog.2015.02.007
Bonkoungou EG (2002) The shea tree (Vitellaria paradoxa) and the African shea parklands. CFC Tech pap 4(21):51–59
Ojo O, Kengne MHK, Fotsing MCF, Mmutlane EM, Ndinteh DT (2021) Traditional uses, phytochemistry, pharmacology and other potential applications of Vitellaria paradoxa Gaertn. (Sapotaceae): A review. Arab J Chem 14:103213
Kuete V (2014) Physical, hematological, and histopathological signs of toxicity induced by African medicinal plants. In: Kuete V (ed) Toxicological survey of African medicinal plants. Elsevier, pp 635–657
Karambiri M, Elias M, Vinceti B, Grosse A (2017) Exploring local knowledge and preferences for shea (Vitellaria paradoxa) ethnovarieties in Southwest Burkina Faso through a gender and ethnic lens. Forests, Trees and Livelihoods 26:13–28. https://doi.org/10.1080/14728028.2016.1236708
Hall JB, Aebischer DP, Tomlinson HF et al (1996) Vitellaria paradoxa. A monograph. University of Wales, Bangor, p 8
Donkor MN, Mosobil R, Abaah EA et al (2021) Potential of shea fruit-based ingredients for the feed industry. Agric Food Secur 10:54. https://doi.org/10.1186/s40066-021-00326-5
Iddrisu A, Zakpaa HD, Mills-Robertson FC, Lowor ST (2020) Vitellaria paradoxa fruit pulp bioethanol production potential: a review. Afr J Biochem Res 14:33–45. https://doi.org/10.5897/AJBR2019.1070
Gyedu-Akoto E, Amon-Armah F, Yabani D (2017) Utilization of shea fruit to enhance food security and reduce poverty in Ghana. Afr J Sci Technol Innov Dev 9:697–705. https://doi.org/10.1080/20421338.2017.1358915
Akoma O, Nma N, Musa S, Salihu A (2018) Nutritional and phytochemical composition of Vitellaria paradoxa (Shea Fruit Pulp). Int J Biochem Res Rev 22:1–7. https://doi.org/10.9734/IJBCRR/2018/41714
Maranz S, Kpikpi W, Wiesman Z et al (2004) Nutritional values and indigenous preferences for shea fruits (vitellaria paradoxa C.F. Gaertn. F.) in African Agroforestry Parklands. Econ Bot 58:588–600. https://doi.org/10.1663/0013-0001(2004)058[0588:NVAIPF]2.0.CO;2
Eromosele IC, Eromosele CO, Kuzhkuzha DM (1991) Evaluation of mineral elements and ascorbic acid contents in fruits of some wild plants. Plant Food Hum Nutr 41:151–154. https://doi.org/10.1007/BF02194083
Ueda JM, Pedrosa MC, Heleno SA et al (2022) Food additives from fruit and vegetable by-products and bio-residues: a comprehensive review focused on sustainability. Sustainability 14:5212. https://doi.org/10.3390/su14095212
Jiménez-Moreno N, Esparza I, Bimbela F, Gandía LM, Ancín-Azpilicueta C (2020) Valorization of selected fruit and vegetable wastes as bioactive compounds: Opportunities and challenges. Crit Rev Environ Sci Technol 50:2061–2108. https://doi.org/10.1080/10643389.2019.1694819
Suleria HAR, Barrow CJ, Dunshea FR (2020) Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peel. Foods 9:1206. https://doi.org/10.3390/foods9091206
Matharu AS, de Melo EM, Houghton JA (2016) Opportunity for high value-added chemicals from food supply chain wastes. Bioresour Technol 215:123–130. https://doi.org/10.1016/j.biortech.2016.03.039
Eldeeb TM, Aigbe UO, Ukhurebor KE et al (2022) Biosorption of acid brown 14 dye to mandarin-CO-TETA derived from mandarin peel. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-02664-1
Gupta G, Baranwal M, Saxena S et al (2022) Utilization of banana waste as a resource material for biofuels and other value-added products. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-02306-6
Ghanem N, Mihoubi D, Bonazzi C et al (2020) Drying characteristics of lemon by-product (Citrus limon. v. lunari): effects of drying modes on quality attributes kinetics’. Waste Biomass Valor 11:303–322. https://doi.org/10.1007/s12649-018-0381-z
Mühlbauer HW, Müller J (2020) Drying Atlas: Drying kinetics and quality of agricultural products. Woodhead Publishing
Paul A, Martynenko A (2021) Electrohydrodynamic drying: effects on food quality. Dry Technol 39:1745–1761. https://doi.org/10.1080/07373937.2021.1906694
Hasan MU, Malik AU, Ali S et al (2019) Modern drying techniques in fruits and vegetables to overcome postharvest losses: a review. J Food Process Preserv 43:e14280. https://doi.org/10.1111/jfpp.14280
Suri S, Singh A, Nema PK (2022) Infrared drying of Kinnow (Citrus reticulata) peel waste: kinetics and quality characterization. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02844-z
Riadh MH, Ahmad SAB, Marhaban MH, Soh AC (2014) Infrared heating in food drying: an overview. Dry Technol 33:322–335. https://doi.org/10.1080/07373937.2014.951124
Ranjan R, Irudayaraj J, Jun S (2002) Simulation of infrared drying process. Dry Technol 20(2):363–379. https://doi.org/10.1081/DRT-120002547
Huang D, Yang P, Tang X, Luo L, Sunden B (2021) Application of infrared radiation in the drying of food products. Trends Food Sci Technol 110:765–777. https://doi.org/10.1016/j.tifs.2021.02.039
Wen Y, Chen L, Li B, Ruan Z, Pan Q (2020) Effect of infrared radiation-hot air ( IR-HA ) drying on kinetics and quality changes of star anise (Illicium verum). Dry Technol 39:90–103. https://doi.org/10.1080/07373937.2019.1696816
Zeng Y, Liu Y, Zhang J, Xi H, Duan X (2019) Effects of far-infrared radiation temperature on drying characteristics, water status, microstructure and quality of kiwifruit slices. J Food Meas Charact 13:3086–3096. https://doi.org/10.1007/s11694-019-00231-3
Adak N, Heybeli N, Ertekin C (2017) Infrared drying of strawberry. Food Chem 219:109–116. https://doi.org/10.1016/j.foodchem.2016.09.103
Xu M, Tian G, Zhao C, Ahmad A, Zhang H, Bi J, Xiao H, Zheng J (2017) Infrared drying as a quick preparation method for dried tangerine peel. International Journal of Analytical Chemistry. https://doi.org/10.1155/2017/6254793
Çağlar A, Toğrul İT, Toğrul H (2009) Moisture and thermal diffusivity of seedless grape under infrared drying. Food Bioprod Process 87:292–300. https://doi.org/10.1016/j.fbp.2009.01.003
Celma AR, López-Rodríguez F, Blázquez FC (2009) Experimental modelling of infrared drying of industrial grape by-products. Food Bioprod Process 87:247–253. https://doi.org/10.1016/j.fbp.2008.10.005
Nowak D, Lewicki PP (2004) Infrared drying of apple slices. Inovative Food Sci Emerg Technol 5:353–360. https://doi.org/10.1016/j.ifset.2004.03.003
Kocabiyik H (2011) Combined infrared and hot air drying. In: Atungulu Pan J, GG, (eds) Infrared heating for food and agricultural processing. CRC Press, pp 101–116
Perez NE, Schmalko ME (2009) Convective drying of pumpkin: influence of pretreatment and drying temperature. J Food Process Eng 32:88–103. https://doi.org/10.1111/j.1745-4530.2007.00200.x
Jain D, Pathare PB (2004) Selection and evaluation of thin layer drying models for infrared radiative and convective drying of onion slices. Biosys Eng 89:289–296. https://doi.org/10.1016/j.biosystemseng.2004.07.011
Akgun NA, Doymaz I (2005) Modelling of olive cake thin-layer drying process. J Food Eng 68:455–461. https://doi.org/10.1016/j.jfoodeng.2004.06.023
Toğrul H (2005) Simple modeling of infrared drying of fresh apple slices. J Food Eng 71:311–323. https://doi.org/10.1016/j.jfoodeng.2005.03.031
Sadeghi E, Asl AH, Movagharnejad K (2019) Mathematical modelling of infrared - dried kiwifruit slices under natural and forced convection. Food Sci Nutr 7:3589–3606. https://doi.org/10.1002/fsn3.1212
Doymaz I, Karasu S, Baslar M (2016) Effects of infrared heating on drying kinetics, antioxidant activity, phenolic content, and color of jujube fruit. J Food Meas Charact 10:283–291. https://doi.org/10.1007/s11694-016-9305-4
Henderson SM, Pabis S (1961) Grain drying theory I: Temperature effect on drying coefficient. J Agric Res Eng 6:169–174
Wang CY, Singh RP (1978) A single layer drying equation for rough rice. ASAE Paper No. 78-3001, St. Joseph, MI, USA
Chayjan RA, Kaveh M, Khayati S (2014) Modeling some drying characteristics of sour cherry (Prunus cerasus L.) under infrared radiation using mathematical models and artificial neural networks. Agric Eng Int: CIGR J 16:265–279
Yaldiz O, Ertekin C (2001) Thin layer solar drying of some vegetables. Dry Technol 19:583–597. https://doi.org/10.1081/DRT-100103936
Delfiya DSA, Prashob K, Murali S, Alfiya PV, Samuel MP, Pandiselvam R (2022) Drying kinetics of food materials in infrared radiation drying: A review. J Food Process Eng 45:1–19. https://doi.org/10.1111/jfpe.13810
Aidani E, Hadadkhodaparast M, Kashaninejad M (2017) Experimental and modeling investigation of mass transfer during combined infrared-vacuum drying of Hayward kiwifruits. Food Sci Nutr 5:596–601. https://doi.org/10.1002/fsn3.435
Doymaz I (2007) The kinetics of forced convective air-drying of pumpkin slices. J Food Eng 79:243–248. https://doi.org/10.1016/j.jfoodeng.2006.01.049
Doymaz İ (2017) Drying kinetics, rehydration and colour characteristics of convective hot-air drying of carrot slices. Heat Mass Transf 53:25–35. https://doi.org/10.1007/s00231-016-1791-8
Singh B, Gupta AK (2007) Mass transfer kinetics and determination of effective diffusivity during convective dehydration of pre-osmosed carrot cubes. J Food Eng 79:459–470. https://doi.org/10.1016/j.jfoodeng.2006.01.073
Korese JK, Achaglinkame MA, Chikpah SK (2021) Effect of hot air temperature on drying kinetics of palmyra (Borassus aethiopum Mart. ) seed-sprout fleshy scale slices and quality attributes of its flour. J Agric Food Res 6:100249. https://doi.org/10.1016/j.jafr.2021.100249
Ghosh U, Gangopadhyay H (2004) Effect of drying methods on rehydration kinetics of potato slices. J Sci Ind Res 63:452–457
Association of Official Analytical Chemists (AOAC) (2012) Official methods of analysis of AOAC International, 19th edn. AOAC International, Gaithersburg, MD, USA
Association of Official Analytical Chemists (AOAC) (2010) Official methods of analysis of AOAC International, 17th edn. AOAC International, Gaithersburg, MD, USA
Izli N, Izli G, Taskin O (2018) Impact of different drying methods on the drying kinetics, color, total phenolic content and antioxidant capacity of pineapple. CYTA - Journal of Food 16:213–221. https://doi.org/10.1080/19476337.2017.1381174
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Meda A, Laien CE, Romito M, Millogo J, Nacoulma OG (2005) Determination of total phenolic, flavonoid and proline contents in Burkina Faso honeys as well as well as their radical scavenging activity. Food Chem 91:571–577. https://doi.org/10.1016/j.foodchem.2004.10.006
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of Vitamin E. Anal Biochem 269:337–341. https://doi.org/10.1006/abio.1999.4019
Praneetpolkrang P, Sathapornprasath K (2021) Thin-layer drying model of jackfruit using artificial neural network in a far infrared dryer. Eng Appl Sci Res 48(2):181–189. https://doi.org/10.14456/easr.2021.20
Zhu A, Shen X (2014) The model and mass transfer characteristics of convection drying of peach slices. Int J Heat Mass Transf 72:345–351. https://doi.org/10.1016/j.ijheatmasstransfer.2014.01.001
Zogzas NP, Maroulis ZB, Marinos-Kouris D (1996) Moisture diffusivity data compilation in foodstuffs. Dry Technol 14:2225–2253. https://doi.org/10.1080/07373939608917205
Łechtanska JM, Szadzinska J, Kowalski SJ (2015) Microwave- and infrared-assisted convective drying of green pepper : quality and energy considerations. Chem Eng Process: Process Intensif J 98:155–164. https://doi.org/10.1016/j.cep.2015.10.001
Diamante L, Durand M, Savage G, Vanhanen L (2010) Effect of temperature on the drying characteristics, colour and ascorbic acid content of green and gold kiwifruits. Int Food Res J 17:441–451
Noomhorm A (2007) Overview of dehydration method on quality of fruit and vegetables. SWU Sci J 23:9–22
Moreira R, Chenlo F, Chaguri L, Fernandes C (2008) Water absorption, texture, and color kinetics of air-dried chestnuts during rehydration. J Food Eng 86:584–594. https://doi.org/10.1016/j.jfoodeng.2007.11.012
Krokida MK, Marinos-Kouris D (2003) Rehydration kinetics of dehydrated products. J Food Eng 57(1):1–7. https://doi.org/10.1016/S0260-8774(02)00214-5
Bikila AM, Tola Y, Esho TB, Forsido SF (2020) Effect of predrying treatment and drying temperature on proximate composition, mineral contents, and thermophysical properties of anchote (Coccinia abyssinica (Lam.) Cogn.) flour. Food Sci Nutr 8:5532–5544. https://doi.org/10.1002/fsn3.1860
Honfo FG, Akissoe N, Linnemann AR, Soumanou M, Van Boekel MAJS (2014) Nutritional composition of shea products and chemical properties of shea butter: a review. Crit Rev Food Sci Nutr 54:673–686. https://doi.org/10.1080/10408398.2011.604142
Wu X, Zhang M, Li Z (2019) Influence of infrared drying on the drying kinetics, bioactive compounds and flavor of Cordyceps militaris. LWT Food Sci Technol 111:790–798. https://doi.org/10.1016/j.lwt.2019.05.108
González S (2020) Dietary bioactive compounds and human health and disease. Nutrients 12:348. https://doi.org/10.3390/nu12020348
Sturm B, Hensel O (2017) Pigments and nutrients during vegetable drying process, dried products storage and their associated colour changes. In: Zhang M Bhandari B Fang Z Handbook of drying of vegetables and vegetable products, 1st edn. CRC Press Taylor & Francis, Boca Raton, pp 257–277
İncedayi B, Tamer CE, Suna SGÖ, Çopur S, ÖU, (2016) Impact of different drying parameters on color, β-carotene, antioxidant activity and minerals of apricot (Prunus armeniaca L.). Food Sci Technol 36:171–178. https://doi.org/10.1590/1678-457X.0086
Sogi DS, Siddiq M, Dolan KD (2015) Total phenolics, carotenoids and antioxidant properties of Tommy Atkin mango cubes as affected by drying techniques. LWT Food Sci Technol 62:564–568. https://doi.org/10.1016/j.lwt.2014.04.015
Erenturk S, Gulaboglu MS, Gultekin S (2005) The effects of cutting and drying medium on the vitamin C content of rosehip during drying. J Food Eng 68:513–518. https://doi.org/10.1016/j.jfoodeng.2004.07.012
Mrad ND, Boudhrioua N, Kechaou N, Courtois F, Bonazzi C (2012) Influence of air drying temperature on kinetics, physicochemical properties, total phenolic content and ascorbic acid of pears. Food Bioprod Process 90:433–441. https://doi.org/10.1016/j.fbp.2011.11.009
Timoumi S, Mihoubi D, Zagrouba F (2007) Shrinkage, vitamin C degradation and aroma losses during infra-red drying of apple slices. LWT Food Sci Technol 40:1648–1654. https://doi.org/10.1016/j.lwt.2006.11.008
Nguyen N-V, Nguyen Q-D, Tran P-B, Huynh B-L, PT, (2020) Effects of drying conditions in low-temperature microwave-assisted drying on bioactive compounds and antioxidant activity of dehydrated bitter melon (Momordica charantia L.). Food Sci Nutr 8:3826–3834. https://doi.org/10.1002/fsn3.1676
Sturm B, Hofacker WC, Hensel O (2012) Optimizing the drying parameters for hot-air-dried apples. Dry Technol 30:1570–1582. https://doi.org/10.1080/07373937.2012.698439
Acknowledgements
The authors would like to acknowledge Mr. Matthew Atongbiik Achaglinkame for his assistance in the preparation of experimental materials.
Funding
This research work was financially supported by the International Foundation for Science [Grant Number I3-E-6582–1].
Author information
Authors and Affiliations
Contributions
Joseph Kudadam Korese: conceptualization, methodology, investigation, funding acquisition, project administration, visualization, formal analysis, writing the original draft; Solomon Kofi Chikpah: formal analysis, writing the original draft. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Korese, J.K., Chikpah, S.K. Understanding infrared drying behavior of shea (Vitellaria paradoxa) fruit by-product for the production of value-added products. Biomass Conv. Bioref. 13, 15001–15015 (2023). https://doi.org/10.1007/s13399-022-03494-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03494-x