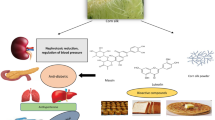
Abstract
Certain population in rural zones are still getting assistance from the traditional and folkloric medical systems. Before they cease to exist, this practice and knowledge need to be documented. Acorus calamus is a well-known aromatic herb with a rhizome that has been exhibited various therapeutic applications like gastroenteritis, antibacterial, antioxidant, antifungal, neuroprotective, antidiabetic, anti-inflammatory, anticancer, and anti-asthmatic activity. In Siddha system, the thermally treated dried rhizome of A. calamus is generally used to cure gastrointestinal and neurological ailments occur especially in infants by mixing the powder of rhizome with milk, ghee, and water. In this study, we evaluate the effect of thermal treatment on screening of bioactive compounds from A. calamus rhizome. The dried rhizome was thermally treated at different temperatures and incubation periods followed by ethanol extraction for 12 h to obtain the bioactive compounds profile. The temperature was optimized to 500 °C for 100 s of incubation based on the number of identified compounds through GC–MS analysis. The optimized samples were further analyzed by using LC–MS/MS, FTIR, micro-Raman, DLS-zeta potential, and XRD. A number of sesquiterpenoids, alkaloids, fatty acids, amino acid derivatives, sterols, and glucosides were detected in the aforementioned methods. The difference was observed in the size and charge of thermally treated and control dried rhizome. This indicates the short time exposure of thermally treated rhizome to be enough to retrieve the major bioactive compounds and also has tremendous application to revert the ailments, over to dried control rhizome.






Similar content being viewed by others
Data availability
Data and materials are available upon request.
References
Dixon RA (2001) Natural products and plant disease resistance. Nature 411(6839):843–847
Mukherjee PK, Kumar V, Mal M, Houghton PJ (2007) Acorus calamus.: scientific validation of ayurvedic tradition from natural resources. Pharmaceutical Biology 45(8):651–666
Berg K, Bischoff R, Stegmüller S, Cartus A, Schrenk D (2016) Comparative investigation of the mutagenicity of propenylic and allylic asarone isomers in the Ames fluctuation assay. Mutagen 443–451. https://doi.org/10.1093/mutage/gew007
Pachaiappan R, Nagasathiya K, Singh PK, Gopalakrishnan AV, Velusamy P, Ramasamy K (2022) Phytochemical profile of black cumin (Nigella sativa L.) seed oil: identification of bioactive anti-pathogenic compounds for traditional Siddha formulation. Bio Conver Biorefin 1–13. https://doi.org/10.1007/s13399-022-02951-x
McGaw LJ, Jäger AK, Van Staden J, Eloff JN (2002) Isolation of β-asarone, an antibacterial and anthelmintic compound, from Acorus calamus in South Africa. S Afric J Bot 68:31–35. https://doi.org/10.1016/S0254-6299(16)30450-1
Sharma V, Singh I, Chaudhary P (2014) Acorus calamus (the healing plant): a review on its medicinal potential, micropropagation and conservation. Nat Prod Res 28:1454–1466. https://doi.org/10.1080/14786419.2014.915827
Alsaud N, Farid M (2020) Insight into the influence of grinding on the extraction efficiency of selected bioactive compounds from various plant leaves. Appl Sci 10:6362. https://doi.org/10.3390/app10186362
Prasedya ES, Frediansyah A, Martyasari NW, Ilhami BK, Abidin AS, Padmi H, Juanssilfero AB, Widyastuti S, Sunarwidhi AL (2021) Effect of particle size on phytochemical composition and antioxidant properties of Sargassum cristaefolium ethanol extract. Scient Rep 11:1–9. https://doi.org/10.1038/s41598-021-95769-y
Zuo HL, Yang FQ, Zhang XM, Xia ZN (2012) Separation of cis-and trans-asarone from Acorus tatarinowii by preparative gas chromatography. J Analyt Meth Chem
Parthasarathy S, Soundararajan P, Krishnan N, Karuppiah KM, Devadasan V, Prabhu D (2022) Detection of adulterants from common edible oils by GC–MS. Bio Conver Bioref 1–21. https://doi.org/10.1007/s13399-022-02913-3
Krishnan N, Mariappanadar V, Dhanabalan AK, Devadasan V, Gopinath SC, Raman P (2022) Purification, identification and in silico models of alkaloids from Nardostachys jatamansi—bioactive compounds for neurodegenerative diseases. Biomass Conversion and Biorefinery 1–12. https://doi.org/10.1007/s13399-022-03237-y
Krishnan N, Raman P, Mariappanadar V (2015) Simple mass spectrometric method for the estimation of boron and aluminum in water at the parts per billion level. Europ J Mas Spec 2:481–486. https://doi.org/10.1255/ejms.1349
Wulandari L, Retnaningtyas Y, Lukman H (2016) Analysis of flavonoid in medicinal plant extract using infrared spectroscopy and chemometrics. J Analyt Meth Chem. https://doi.org/10.1155/2016/4696803
Zeise I, Heiner Z, Holz S, Joester M, Büttner C, Kneipp J (2018) Raman imaging of plant cell walls in sections of Cucumis sativus. Plant 7:7. https://doi.org/10.3390/plants7010007
Hunter RJ (1981) Zeta potential in colloid science: Principles and Applications. Academic press, London
Mani S, Bhatt SB, Vasudevan V et al (2022) The updated review on plant peptides and their applications in human health. Int J Pept Res Ther 28:135. https://doi.org/10.1007/s10989-022-10437-7
Kenes K, Yerdos O, Zulkhair M, Yerlan D (2012) Study on the effectiveness of thermally treated rice husks for petroleum adsorption. J Non-Cryst Solids 358:2964–2969
Venkateswaran V, Muralidharan S, Gopalakrishnan AV, Krishnan N, Velmurugan D, Raman P (2021) GC-MS analysis of guttation fluids from selected crop plants. J Crop Improv 36(6):801–815. https://doi.org/10.1080/15427528.2021.2018748
Oprean R, Tamas M, Roman L (1998) Comparison of GC-MS and TLC techniques for asarone isomers determination. J Pharmaceu Biomed Analys 18:227–234. https://doi.org/10.1016/s0731-7085(98)00161-7
Shi C, Shi YQ (2006) Research progress of asarone. Chin Archiv Trad Chines Med 24:1285–1287
Unger P, Melzig MF (2012) Comparative study of the cytotoxicity and genotoxicity of alpha-and beta-asarone. Scient Pharmaceut 80:663–668. https://doi.org/10.3797/scipharm.1204-21
Mohar BO, Espinoza-Aguirre C, Cortinas dew NavChamorro G (1986) Determinación de la actividad mutagénica de alpha-asarona en ei sistema Salmonella typhimurium/microsomas. In Proceedings of 1st Congress of the Mexican Association of Mutagenesis, Carcinogenesis and Teratogenesis
Cho J, Kim YH, Kong JY, Yang CH, Park CG (2002) Protection of cultured rat cortical neurons from excitotoxicity by asarone, a major essential oil component in the rhizomes of Acorus gramineus. Life Sci 71:591–599. https://doi.org/10.1016/s0024-3205(02)01729-0
Zou DJ, Wang G, Liu JC, Dong MX, Li XM, Zhang C, Zhou L, Wang R, Niu YC (2011) Beta-asarone attenuates beta-amyloid-induced apoptosis through the inhibition of the activation of apoptosis signal-regulating kinase 1 in SH-SY5Y cells. Die Pharmazie- Int J Pharmaceu Sci 66:44–51
Manikandan S, Devi RS (2005) Antioxidant property of α-asarone against noise-stress-induced changes in different regions of rat brain. Pharmacol Res 52:467–474
Balakrishnan R, Cho DY, Kim IS, Seol SH, Choi DK (2022) Molecular mechanisms and therapeutic potential of α-and β-asarone in the treatment of neurological disorders. Antioxidants 11(2):281
Hei X, Xie M, Xu J, Li J, Liu T (2020) β-Asarone exerts antioxidative effects on H2O2-stimulated PC12 cells by activating Nrf2/HO-1 pathway. Neurochem Res 45(8):1953–1961
Chang W, Teng J (2015) β-asarone prevents Aβ25-35-induced inflammatory responses and autophagy in SH-SY5Y cells: down expression beclin-1, LC3B and up expression Bcl-2. Int J Clin Exp Med 8(11):20658
Ellervik C, Mandrup-Poulsen T, Nordestgaard BG, Larsen LE, Appleyard M, Frandsen et al (2001) Prevalence of hereditary haemochromatosis in late-onset type 1 diabetes mellitus: a retrospective study. The Lancet 358(9291):1405–1409
Roetto A, Bosio S, Gramaglia E, Barilaro MR, Zecchina G, Camaschella C (2002) Pathogenesis of hyperferritinemia cataract syndrome. Blood Cells Mol Dis 29(3):532–535
Valdes AM, Walter J, Segal E, Spector TD (2018) Role of the gut microbiota in nutrition and health. BMJ 361:k2179
Liu S, Moulton KR, Auclair JR, Zhou ZS (2016) Mildly acidic conditions eliminate deamidation artifact during proteolysis: digestion with endoprotease Glu-C at pH 4.5. Amino Acids 48(4):1059–1067
Garcia C, Antona C, Robert B, Lopez C, Armand M (2014) The size and interfacial composition of milk fat globules are key factors controlling triglycerides bioavailability in simulated human gastro-duodenal digestion. Food Hydrocolloids 35:494–504
Heinlein A, Buettner A (2012) Monitoring of biotransformation of hop aroma compounds in an in vitro digestion model. Food Funct 3(10):1059–1067
Calenic B, Miricescu D, Greabu M, Kuznetsov AV, Troppmair J, Ruzsanyi V, Amann A (2015) Oxidative stress and volatile organic compounds: interplay in pulmonary, cardio-vascular, digestive tract systems and cancer. Open Chemistry 13(1):1020–1030
ÖzkanKarabacak A, Özoğlu Ö, Durgut S et al (2021) Development of purple basil (Ocimum basilicum L.) sherbet fortified with propolis extract using response surface methodology. Food Measure 15:4972–4991. https://doi.org/10.1007/s11694-021-01064-9
Adams TB, Doull J, Goodman JI, Munro IC, Newberne P, Portoghese PS, Ford RA (1997) The FEMA GRAS assessment of furfural used as a flavour ingredient. Food Chem Toxicol 35(8):739–751
Andrews JM, Roman DP Jr, Bing DH, Cory M (1978) Inhibition of four human serine proteases by substituted benzamidines. J Med Chem 21(12):1202–1207
Cui JL, Gao XY, Vijayakumar V, Guo ZX, Wang ML, Wang JH, Liu L (2020) Regulation by fungal endophyte of Rhodiola crenulata from enzyme genes to metabolites based on combination of transcriptome and metabolome. J Sci Food Agric 100(12):4483–4494
Cheah IK, Halliwell B (2021) Ergothioneine, recent developments. Redox Biol 42:101868
Song T-Y, Chen C-L, Liao J-W, Ou H-C, Tsai M-S (2010) Ergothioneine protects against neuronal injury induced by cisplatin both in vitro and in vivo. Food Chem Toxicol 48(12):3492–3499
Whitley R, Tucker B, Kinkel A, Barton N, Pass R, Whelchel J, Cobbs C, Diethelm A, Buchanan R (1980) Pharmacology, tolerance, and antiviral activity of vidarabine monophosphate in humans. Antimicrob Agents Chemother 18:709
Tejada S, Sureda A, Roca C, Gamundi A, Esteban S (2007) Antioxidant response and oxidative damage in brain cortex after high dose of pilocarpine. Brain Res Bull 71(4):372–375
Kartikeyan A, Vasudevan V, Peter AJ et al (2022) Effect of incubation period on the glycosylated protein content in germinated and ungerminated seeds of mung bean (Vigna radiata (L.) Wilczek). Int J Biol Macromol 30:633. https://doi.org/10.1016/j.ijbiomac.2022.07.036
Yu F, Feng X, Li X, Luo Y, Wei M, Zhao T, Xia J (2021) Gut-derived metabolite phenylacetylglutamine and white matter hyperintensities in patients with acute ischemic stroke. Front Aging Neurosci 13
Pachaiappan R, Rajamuthu TP, Sarkar A, Natrajan P, Krishnan N, Sakthivelu M (2022) N-acyl-homoserine lactone mediated virulence factor (s) of Pseudomonas aeruginosa inhibited by flavonoids and isoflavonoids. Process Biochem 116:84–93
Santarelli F, Cassanello M, Enea A, Poma F, D’Onofrio V, Guala G, Garrone G, Puccinelli P, Caruso U, Porta F, Spada M (2013) A neonatal case of 3-hydroxy-3-methylglutaric-coenzyme A lyase deficiency. Ital J Pediatr 24(39):33. https://doi.org/10.1186/1824-7288-39-33
Wang D, Li W, Zou Q, Yin L, Du Y, Gu J, Suo J (2017) Serum metabolomic profiling of human gastric cancer and its relationship with the prognosis. Oncotarget 8(66):110000
Jaiswal P, Kumar P, Singh VK, Singh DK (2009) Biological effects of Myristicafragrans. Annu Rev Biomed Sci 11:21–29. https://doi.org/10.5016/1806-8774.2009v11p21
Martel JL, Kerndt CC, Doshi H, Franklin DS (2021) Vitamin B1 (thiamine). In Stat Pearls [Internet]. Stat Pearls Publishing
Krishnan N, Devadasan V, Raman P (2020) Plant-derived alkaloids as anti-viral agents. Int J Res Pharma Sci 6174–6182. https://doi.org/10.26452/ijrps.v11i4.3291
Boulghobra D, Grillet PE, Laguerre M, Tenon M, Fauconnier J, Fança-Berthon P, Cazorla O (2020) Sinapine, but not sinapic acid, counteracts mitochondrial oxidative stress in cardiomyocytes. Redox Biol 34:101554
Muriithi MW, Abraham WR, Addae-Kyereme J, Scowen I, Croft SL, Gitu PM, ..., Wright CW (2002) Isolation and in vitro Antiplasmodial Activities of Alkaloids from Tecleat richocarpa: In vivo Antimalarial Activity and X-ray Crystal Structure of Normelicopicine. J Nat prod 65(7):956–9
Shi X, Zhu M, Kang Y, Yang T, Chen X, Zhang Y (2018) Wnt/β-catenin signaling pathway is involved in regulating the migration by an effective natural compound brucine in LoVo cells. Phytomedicine 46:85–92. https://doi.org/10.1016/j.phymed.2018.04.019
Phillipson JD, Hemingway SR (1975) Chromatographic and spectroscopic methods for the identification of alkaloids from herbarium samples of the genus Uncaria. J Chromatogr A 105(1):163–178
Saad MFM, Goh HH, Rajikan R, Yusof TRT, Baharum SN, Bunawan H (2020) Uncaria gambir (W Hunter) Roxb: from phytochemical composition to pharmacological importance. Trop J Pharm Res 19(8):1767–1773
Marlies EHM, Van Hoef (2019) Cladribine in the treatment of Langerhans cell histocytosis: a summary of the literature. J Rare Disord: Diagn Ther 1–15
Gudi G, Krahmer A, Kruger H, Henning L, Schulz H (2014) Discrimination of fennel chemotypes applying IR and Raman spectroscopy Discovery of a new γ-asarone chemotype. J Agric Food Chem 62:3537–3547
Wang LH, Chen JX, Wang CC (2014) Rapid quantitative analysis of suspected fragrance allergens in between commercial essential oils and using attenuated total reflectance–infrared (ATR–IR) spectroscopy. J Essent Oil Res 26(3):185–196
Chen M, Zeng H, Larkum AW, Cai ZL (2004) Raman properties of chlorophyll d, the major pigment of Acaryochloris marina: studies using both Raman spectroscopy and density functional theory. Spectrochim Acta Part A Mol Biomol Spectrosc 60(3):527–534
Pruteanu A, David L, Matache M, Nitu M (2018) Particle size distribution of some chopped medicinal plants. In 17th International Scientific Conference Engineering for Rural Development Proceedings 17:640–645
Carye OJ, Noble PF, Paunov (2004) Fabrication of novel colloidosome microcapsules with gelled aqueous cores. J Mater Chem 14(22):3351–3355
Belarmino DD, Ladchumananandasivam R, Belarmino LD, Pimentel JR, da Rocha BG, Galv AO, de Andrade SM (2012) Physical and morphological structure of chicken feathers (keratin biofiber) in natural, chemically and thermally modified forms. Mate Scie and Appli 3(12):87–893
Poralan GM, Gambe JE, Alcantara EM, Vequizo RM (2015) X-ray diffraction and infrared spectroscopy analyses on the crystallinity of engineered biological hydroxyapatite for medical application. In IOP Conf Ser: Mater Sci Eng 79(1):012028
Acknowledgements
The authors wish to express their sincere thanks to the management of SRM Institute of Science and Technology and Department of Biotechnology, School of Bioengineering for the research facilities to carry out FTIR, GC-MS, and micro-Raman facilities used in this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: RP; methodology: NK and PKS; formal analysis and investigation: NK, PKS, and MS; writing — original MS draft: NK, PKS, and MS; writing — review and editing: PV, SCBG, and RP.
Corresponding authors
Ethics declarations
Ethical approval
In this study, we have not used human or animal studies.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krishnan, N., Singh, P.K., Sakthivelu, M. et al. Influence of thermal treatment on extraction and characteristics of phytochemicals from rhizome of Acorus calamus L. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03415-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03415-y