Abstract
Zanthoxylum branch became agricultural waste without proper utilization, and the pristine biochar (PB) had the limited removal capacity of Cr(VI) (21.75 mg g−1). In this study, zanthoxylum branch was fabricated to modified biochar (MZB) through impregnating with 0.4, 0.6, 0.8, and 1.0 mol L−1 Fe (NO3)3 solution and then pyrolyzing at 500 and 900 ℃. After sieving by batch adsorption experiment of Cr(VI), the optimal MZB was prepared with impregnating solution concentration of 0.4 mg L−1 and pyrolysis temperature of 900 ℃ (900MZB4), and displayed the outstanding Cr(VI) removal capacity (110.43 mg g−1). Besides, the effect of initial solution pH was evaluated, and removal process of Cr(VI) was a reaction of consuming protons. The fitting results of Langmuir model and pseudo-second-order kinetic model confirmed that the Cr(VI) removal reaction was a homogeneous chemisorption. Thermodynamic parameters demonstrated that the adsorption of Cr(VI) was an endothermic spontaneous process. The results of SEM and XRD suggested that zero-valent iron (Fe0) particles were located on 900MZB4 surface and participated in the removal reaction of Cr(VI). The XPS analysis indicated 78.37% of Cr(VI) was reduced to Cr(III) by the cooperation of oxygen-containing functional groups (OFG) and Fe0. More OFG and higher surface area (130.36 m2 g−1) revealed by FTIR and BET also contributed to the better characteristic of 900MZB4. In addition, 900MZB4 maintained removal efficiency at 81.22% after sixth regeneration cycles. Therefore, it is feasible to prepare high-efficient adsorbent from zanthoxylum branch for wastewater treatment.
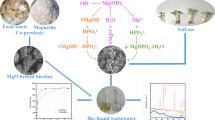
Graphical abstract









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Biores Technol 98:2243–2257. https://doi.org/10.1016/j.biortech.2005.12.006
Aminul Islam M, Angove M, Morton D (2019) Recent innovative research on chromium (VI) adsorption mechanism. Environ Nanotechnol Monit Manage 12:100267. https://doi.org/10.1016/j.enmm.2019.100267
Asri M, Ghachtouli N, Elabed S, Koraichi IS, Bruna A (2018) Wicherhamomyces anomalus biofilm supported on wood husk for chromium wastewater treatment. J Hazard Mater 359:554–562. https://doi.org/10.1016/j.jhazmat.2018.05.050
Demirbas A (2008) Heavy metal adsorption onto agro-based waste materials: a review. J Hazard Mater 157:220–229. https://doi.org/10.1016/j.jhazmat.2008.01.024
Dong FX, Yan L, Zhou XH, Huang ST, Liang JY, Zhang WX, Guo ZW, Guo PR, Qian W, Kong LJ, Chu W, Diao ZH (2021) Simultaneous adsorption of Cr(VI) and phenol by biochar-based iron oxide composites in water: performance, kinetics and mechanism. J Hazard Mater 416:125930. https://doi.org/10.1016/j.jhazmat.2021.125930
Dong H, Deng J, Xie Y, Zhang C, Jiang Z, Cheng Y, Hou K, Zeng G (2017) Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J Hazard Mater 332:79–86. https://doi.org/10.1016/j.jhazmat.2017.03.002
Fu FY, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
He R, Yuan XZ, Huang ZL, Wang H, Jiang LB, Huang J, Tan MJ, Li H (2019) Activated biochar with iron-loading and its application in removing Cr (VI) from aqueous solution. Colloids Surf A 579:123642. https://doi.org/10.1016/j.colsurfa.2019.123642
Imran M, Khan Z, Iqbal MM, Iqbal J, Rizwan M (2020) Effect of biochar modified with magnetite nanoparticles and HNO3 for efficient removal of Cr(VI) from contaminated water: a batch and column scale study. Environ Pollut 261:114231. https://doi.org/10.1016/j.envpol.2020.114231
Inyang M, Gao B, Ying Y, Xue YW, Zimmerman AR, Pullammanappallil P, Cao XD (2012) Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Biores Technol 110:50–56. https://doi.org/10.1016/j.biortech.2012.01.072
Iqbal J, Shah NS, Sayed M, Niazi NK, Imran M, Howari F (2020) Nano-zerovalent manganese/biochar composite for the adsorptive and oxidative removal of Congo-red dye from aqueous solutions. J Hazard Mater 403:123854. https://doi.org/10.1016/j.jhazmat.2020.123854
Karunanayake A, Todd O, Crowley M, Ricchetti L, Pittman C, Anderson R, Mohan D, Mlsnaa T (2018) Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chem Eng J 331:480–491. https://doi.org/10.1016/j.cej.2017.08.124
Li HB, Dong XL, da Silva EB, de Oliveira LM, Chen YS, Ma LQ (2017) Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 178:466–478. https://doi.org/10.1016/j.chemosphere.2017.03.072
Liang LP, Guan XH, Zhong S, Li JL, Wu YL, Tratnyek PG (2014) Coupled effects of aging and weak magnetic fields on sequestration of selenite by zero-valent iron. Environ Sci Technol 48:6326–6334. https://doi.org/10.1021/es500958b
Liu N, Zhang Y, Chao X, Liu P, Lv J, Liu Y, Wang Q (2020) Removal mechanisms of aqueous Cr(VI) using apple wood biochar: a spectroscopic study. J Hazard Mater 384:121371. https://doi.org/10.1021/es500958b
Luo Q, Huang X, Luo Y, Yuan H, Ren T, Li X, Xu D, Guo X, Wu Y (2020) Fluorescent chitosan-based hydrogel incorporating titanate and cellulose nanofibers modified with carbon dots for adsorption and detection of Cr(VI). Chem Eng J 407:127050. https://doi.org/10.1016/j.cej.2020.127050
Marques Neto O, Bellato C, Silva D (2018) Iron oxide/carbon nanotubes/chitosan magnetic composite film for chromium species removal. Chemosphere 218:391–401. https://doi.org/10.1016/j.chemosphere.2018.11.080
Mukhopadhyay B, Sundquist J, Schmitz R (2007) Removal of Cr(VI) from Cr-contaminated groundwater through electrochemical addition of Fe(II). J Environ Manage 82:66–76. https://doi.org/10.1016/j.jenvman.2005.12.005
Qiu B, Wang Y, Sun D, Qiang W, Xin Z, Weeks BL, O’Connor R, Huang X, Wei S, Guo Z (2015) Cr(VI) removal by magnetic carbon nanocomposites derived from cellulose at different carbonization temperatures. J Mater Chem A 3. https://doi.org/10.1016/j.biortech.2016.04.093
Qu JH, Wang YX, Tian X, Jiang Z, Deng FX, Tao Y, Jiang Q, Wang L, Zhang Y (2021) KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: Affecting factors, mechanisms and reusability exploration. J Hazard Mater 401:123292. https://doi.org/10.1016/j.jhazmat.2020.123292
Rajapaksha A, Samrat Alam M, Chen N, Alessi D, Igalavithana A, Tsang D-C-W, Ok Y (2018) Removal of hexavalent chromium in aqueous solutions using biochar: chemical and spectroscopic investigations. Sci Total Environ 625:1567–1573. https://doi.org/10.1016/j.scitotenv.2017.12.195
Rout PR, Bhunia P, Dash RR (2014) Modeling isotherms, kinetics and understanding the mechanism of phosphate adsorption onto a solid waste: ground burnt patties. J Environ Chem Eng 2:1331–1342. https://doi.org/10.1016/j.jece.2014.04.017
Subedi N, Lähde A, Abu-Danso E, Iqbal J, Bhatnagar A (2019) A comparative study of magnetic chitosan (Chi@Fe3O4) and graphene oxide modified magnetic chitosan (Chi@Fe3O4GO) nanocomposites for efficient removal of Cr(VI) from water. Int J Biol Macromol 137:948–959. https://doi.org/10.1016/j.ijbiomac.2019.06.151
Sun W, Xia J, Li S, Sun F (2012) Effect of natural organic matter (NOM) on Cu(II) adsorption by multi-walled carbon nanotubes: Relationship with NOM properties. Chem Eng J 200–202:627–636. https://doi.org/10.1016/j.cej.2012.06.118
Tan XF, Liu YG, Gu YL, Yan X, Li J (2016) Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour Technol 212:318–333. https://doi.org/10.1016/j.biortech.2016.04.093
Tang J, Zhao B, Lyu H, Li D (2021) Development of a novel pyrite/biochar composite (BM-FeS2@BC) by ball milling for aqueous Cr(VI) removal and its mechanisms. J Hazard Mater 413:125415. https://doi.org/10.1016/j.jhazmat.2021.125415
Thangagiri B, Sakthivel A, Jeyasubramanian K, Seenivasan S, Yun K (2021) Removal of hexavalent chromium by biochar-derived from Azadirachta indica leaves: batch and column studies. Chemosphere 286:131598. https://doi.org/10.1016/j.chemosphere.2021.131598
Trinh V, Phuong Nguyen T, Van H, Hoang L, Nguyen T, Ha L-T-H, Vu X, Pham T-T, Nguyen T, Quang N-V, Nguyen X (2020) Phosphate adsorption by silver nanoparticles-loaded activated carbon derived from tea residue. Sci Rep 10:3634. https://doi.org/10.1038/s41598-020-60542-0
Üzüm Ç, Shahwan T, Eroğlu A-E, Hallam K-R, Scott T-B, Lieberwirth I (2009) Synthesis and characterization of kaolinite-supported zero-valent iron nanoparticles and their application for the removal of aqueous Cu2+ and Co2+ ions. Appl Clay Sci 43:172–181. https://doi.org/10.1016/j.clay.2008.07.030
Verma R, Maji PK, Sarkar S (2022) Synthesis and validation of polystyrene-based polyethylenimine composite for Cr(VI) removal from aqueous solution: Performance and mechanism. J Environ Chem Eng 10:107119. https://doi.org/10.1016/j.jece.2021.107119
Wan SL, Wu JY, Zhou SS, Wang R, Gao B, He F (2018) Enhanced lead and cadmium removal using biochar-supported hydrated manganese oxide (HMO) nanoparticles: behavior and mechanism. Sci Total Environ 616–617:1298–1306. https://doi.org/10.1016/j.scitotenv.2017.10.188
Wang J, Chen BL (2015) Adsorption and coadsorption of organic pollutants and a heavy metal by graphene oxide and reduced graphene materials. Chem Eng J 281:379–388. https://doi.org/10.1016/j.cej.2015.06.102
Wang Y, Yu L, Wang R, Wang Y, Zhang X (2020) A novel cellulose hydrogel coating with nanoscale Fe0 for Cr(VI) adsorption and reduction. Sci Total Environ 726:138625. https://doi.org/10.1016/j.scitotenv.2020.138625
Yang Y, Zhang YH, Wang GY, Xian JR, Yang YX, Li T, Pu YL, Jia YX, Li Y, Cheng Z, Zhang SR, Xu XX (2021) Adsorption and reduction of Cr(VI) by a novel nanoscale FeS/chitosan/biochar composite from aqueous solution. J Environ Chem Eng 9:105407. https://doi.org/10.1016/j.jece.2021.105407
Zhang H, Xiao R, Li RH, Ali A, Chen A, Zhang ZQ (2020) Enhanced aqueous Cr(VI) removal using chitosan-modified magnetic biochars derived from bamboo residues. Chemosphere 261:127694. https://doi.org/10.1016/j.chemosphere.2020.127694
Zhang MM, Liu YG, Li TT, Xu WH, Zheng BH, Tan XF, Hui W, Guo YM, Guo FY, Wang SF (2015) Chitosan modification of magnetic biochar produced from Eichhornia crassipes for enhanced sorption of Cr(VI) from aqueous solution. RSC Adv 5:46955–46964. https://doi.org/10.1039/C5RA02388B
Zhang WX, Li XQ, Cao JS (2009) Reply to “Comments on ‘Stoichiometry of Cr(VI) immobilization using nanoscale zerovalent iron (nZVI): a study with high-resolution x-ray photoelectron spectroscopy (HR-XPS).’” Ind Eng Chem Res 48:2298. https://doi.org/10.1021/ie8016434
Zhao N, Yin Z, Liu F, Zhang MY, Lv YZ (2018) Environmentally persistent free radicals mediated removal of Cr(VI) from highly saline water by corn straw biochars. Bioresour Technol 260:294–301. https://doi.org/10.1016/j.biortech.2018.03.116
Zhong X, Lu ZP, Liang W, Hu BW (2020) The magnetic covalent organic framework as a platform for high-performance extraction of Cr(VI) and bisphenol a from aqueous solution. J Hazard Mater 393:122353. https://doi.org/10.1016/j.jhazmat.2020.122353
Zhou L, Chi TY, Zhou YY, Lv JD, Chen H, Sun SQ, Zhu XF, Wu HP, Hu X (2022) Efficient removal of hexavalent chromium through adsorption-reduction-adsorption pathway by iron-clay biochar composite prepared from Populus nigra. Sep Purif Technol 285:120386. https://doi.org/10.1016/j.seppur.2021.120386
Zhou L, Liu Y, Liu S, Yin Y, Zeng G, Tan X, Hu X, Hu X, Jiang L, Ding Y (2016) Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour Technol 218:351–359. https://doi.org/10.1016/j.biortech.2016.06.102
Zhou L, Liu YG, Liu SB, Yin YC, Zeng GM, Tan XF, Hu X, Hu XJ, Jiang LH, Ding Y, Liu SH, H XX (2016) Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour Technol 218:351–359https://doi.org/10.1016/j.biortech.2016.06.102
Zhu SS, Huang XC, Wang DW, Wang L, Fang M (2018) Enhanced hexavalent chromium removal performance and stabilization by magnetic iron nanoparticles assisted biochar in aqueous solution: Mechanisms and application potential. Chemosphere 207:50–59. https://doi.org/10.1016/j.chemosphere.2018.05.046
Acknowledgements
We are very grateful to Q Qu, XH Guo, ZJ Shao, XZ Wang, and MQ Zhu for their insightful suggestions and comments on this paper.
Funding
This study was supported by the National Natural Science Foundation of China (31900105, 51576167).
Author information
Authors and Affiliations
Contributions
Q Qu experimented, analyzed data, and wrote original draft; XH Guo designed experiment, supervised, and financially supported the initial study; ZJ Shao and XZ Wang supervised the field trial; MQ Zhu investigated and disposed the data; L Qiu reviewed, edited the manuscript, designed experiment, and financially supported the primary study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiang Qu and Xiaohui contributed equally to this work and should be considered co-first authors.
Highlights
1. Iron-containing solution concentration and pyrolysis temperature has synergistic effect on iron-oxide form.
2. Zero-valent iron particle was located uniformly on the magnetic biochar surface.
3. The removal capacity by 900MZB4 predicted by Langmuir model was 140.51 mg g−1.
4. Cr(VI) removal process of 900MZB4 was the combination of several mechanisms.
5. Physical adsorption and chemical redox reaction jointly devoted to the Cr(VI) removal.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qu, Q., Guo, X., Shao, Z. et al. Adsorption performance and mechanism of Fe-loaded biochar derived from waste zanthoxylum branch for removing Cr(VI) from aqueous solution. Biomass Conv. Bioref. 14, 10201–10215 (2024). https://doi.org/10.1007/s13399-022-03213-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03213-6