Abstract
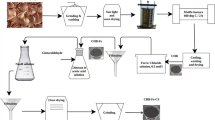
Although waste treatment using photosynthetic bacteria provides beneficial products, the use of free cells limits product recovery and biomass recycling. This research demonstrated the recycling efficiency of Rhodopseudomonas faecalis PA2 immobilized in Ca-alginate for waste cooking oil removal and value-added product recovery and provided a strategy to improve alginate bead stability. Oil removal by immobilized bacteria increased during recycling, but the beads cracked after 3 cycles. To improve bead stability and recycling time, the used beads were immersed in 2% CaCl2 for 24 h before use in each cycle to facilitate Ca2+ coordination in the alginate. After 10 cycles (90 days), the treated beads showed no disintegration and removed 76.26% oil, an increase of 53.87% over single-use beads. The scanning electron microscopic images revealed no surface damage; the internal pores of the cross-section bead where bacteria resided remained unchanged, whereas the untreated bead was destroyed. Energy-dispersive X-ray analysis showed an increase in Ca2+ in the treated beads, indicating Ca2+ absorption during bead immersion. The recovery of value-added products after 10 treatment cycles demonstrated an increase in biomass, protein, and carotenoids by 554.05%, 68.38%, and 2,719.10%, respectively, compared to that for single-use immobilized bacteria. The proposed method to enhance alginate bead recycling, as well as photosynthetic bacteria immobilized in alginate beads, could be a viable alternative for the long-term treatment of waste cooking oil.






Similar content being viewed by others
References
Del Mundo DMN, Sutheerawattananonda M (2017) Influence of fat and oil type on the yield, physico-chemical properties, and microstructure of fat, oil, and grease (FOG) deposits. Water Res 124:308–319. https://doi.org/10.1016/j.watres.2017.07.047
Okino-Delgado CH, Prado DZd, Facanali R, Marques MMO, Nascimento AS, Fernandes CJdC, Zambuzzi WF, Fleuri LF (2017) Bioremediation of cooking oil waste using lipases from wastes. PLoS ONE. https://doi.org/10.1371/journal.pone.0186246
Lopes M, Miranda SM, Belo I (2020) Microbial valorization of waste cooking oils for valuable compounds production – a review. Crit Rev Environ Sci Technol 50:2583–2616. https://doi.org/10.1080/10643389.2019.1704602
Kim JH, Oh YR, Hwang J, Kang J, Kim H, Jang YA, Lee SS, Hwang SY, Park J, Eom GT (2021) Valorization of waste-cooking oil into sophorolipids and application of their methyl hydroxyl branched fatty acid derivatives to produce engineering bioplastics. Waste Manag 124:195–202. https://doi.org/10.1016/j.wasman.2021.02.003
Zhang W, Ji H, Song Y, Ma S, Xiong W, Chen C, Chen B, Zhang X (2020) Green preparation of branched biolubricant by chemically modifying waste cooking oil with lipase and ionic liquid. J Clean Prod 274:122918. https://doi.org/10.1016/j.jclepro.2020.122918
Chaiyarat A, Saejung C (2022) Photosynthetic bacteria with iron oxide nanoparticles as catalyst for cooking oil removal and valuable products recovery with heavy metal co-contamination. Waste Manage 140:81–89. https://doi.org/10.1016/j.wasman.2022.01.005
Zhan P, Liu W (2013) Use of fluidized bed biofilter and immobilized Rhodopseudomonas palustris for ammonia removal and fish health maintenance in a recirculation aquaculture system. Aquaculture Res 44:327–334. https://doi.org/10.1111/j.1365-2109.2011.03038.x
Chen Y, Zhang G, Wang H (2020) Enhancement of photosynthetic bacteria biomass production and wastewater treatment efficiency by zero-valent iron nanoparticles. J Biosci Bioeng 130:306–310. https://doi.org/10.1016/j.jbiosc.2020.04.004
Meng F, Yang A, Wang H, Zhang G, Li X, Zhang Y, Zou Z (2018) One-step treatment and resource recovery of high-concentration non-toxic organic wastewater by photosynthetic bacteria. Bioresour Technol 251:121–127. https://doi.org/10.1016/j.biortech.2017.12.002
Saejung C, Chanthakhot T (2021) Single-phase and two-phase cultivations using different light regimes to improve production of valuable substances in the anoxygenic photosynthetic bacterium Rhodopseudomonas faecalis PA2. Bioresour Technol 328:124855. https://doi.org/10.1016/j.biortech.2021.124855
Higuchi-Takeuchi M, Morisaki K, Numata K (2016) A screening method for the isolation of polyhydroxyalkanoate-producing purple non-sulfur photosynthetic bacteria from natural seawater. Front Microbiol 7:1509
Saejung C, Raksapon D, Chaiyarat A (2022) Synthesis and application of a magnetically recoverable biological nanocomposite material for waste cooking oil removal and commercial product recovery in wastewater treatment. J Clean Prod 363:132425. https://doi.org/10.1016/j.jclepro.2022.132425
Xie GJ, Liu BF, Ding J, Xing DF, Ren HY, Guo WQ, Ren NQ (2012) Enhanced photo-H2 production by Rhodopseudomonas faecalis RLD-53 immobilization on activated carbon fibers. Biomass Bioenergy 44:122–129. https://doi.org/10.1016/j.biombioe.2012.05.002
du Toit JP, Pott RWM (2020) Transparent polyvinyl-alcohol cryogel as immobilisation matrix for continuous biohydrogen production by phototrophic bacteria. Biotechnol Biofuels 13:105. 3:105. https://doi.org/10.1186/s13068-020-01743-7
Segale L, Giovannelli L, Mannina P, Pattarino F (2016) Calcium alginate and calcium alginate-chitosan beads containing celecoxib solubilized in a self-emulsifying phase. Scientifica (Cairo) 2016:5062706. https://doi.org/10.1155/2016/5062706
Duarte JC, Rodrigues JA, Moran PJ, Valenca GP, Nunhez JR (2013) Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Express 3:31. https://doi.org/10.1186/2191-0855-3-31
Kushalatha M, Karigar CS (2018) Biodegradation of halogenated phenol by immobilized phototrophic bacterium Rhodopseudomonas palustris. IJESR 6:75–85
Willaert R, Baron G (1993) Growth kinetics of gel-immobilized yeast cells studied by on-line microscopy. Appl Microbiol Biotechnol 39:347–352. https://doi.org/10.3168/jds.S0022-0302(01)74572-9
Oyeagu U, Nwuche C, Ogbonna C, Ogbonna J (2018) Addition of fillers to sodium alginate solution improves stability and immobilization capacity of the resulting calcium alginate beads. Iran J Biotech. https://doi.org/10.21859/ijb.1824
Klinkenberg G, Lystad KQ, Levine DW, Dyrset N (2001) Cell release from alginate immobilized Lactococcus lactis ssp. lactis in chitosan and alginate coated beads. J Dairy Sci 84:1118–1127. https://doi.org/10.3168/jds.S0022-0302(01)74572-9
Soo CL, Chen CA, Bojo O, Hii YS (2017) Feasibility of marine microalgae immobilization in alginate bead for marine water treatment: Bead stability, cell growth, and ammonia removal. Int J Polym Sci 2017:1–7. https://doi.org/10.1155/2017/6951212
Tomović Nataša S, Trifković Kata T, Rakin Marko P, Rakin Marica B, Bugarski Branko M (2015) Influence of compression speed and deformation percentage on mechanical properties of calcium alginate particles. Chem Ind Chem Eng 21:411–417. https://doi.org/10.2298/CICEQ140228043T
Warburg O, Christian W (1942) Isolation and crystallization of enolase. Biochem Z 310:384–421
Saejung C, Puensungnern L (2020) Evaluation of molasses-based medium as a low cost medium for carotenoids and fatty acid production by photosynthetic bacteria. Waste Biomass Valor 11:143–152. https://doi.org/10.1007/s12649-018-0379-6
Tzirita M, Papanikolaou S, Chatzifragkou A, Quilty B (2018) Waste fat biodegradation and biomodification by Yarrowia lipolytica and a bacterial consortium composed of Bacillus spp. and Pseudomonas putida. Eng Life Sci 18:932–942. https://doi.org/10.1002/elsc.201800067
Laranja JLQ, Ludevese-Pascual GL, Amar EC, Sorgeloos P, Bossier P, De Schryver P (2014) Poly-β-hydroxybutyrate (PHB) accumulating Bacillus spp. Improve the survival, growth and robustness of Penaeus monodon (Fabricius, 1798) postlarvae. Vet Microbiol 173:310–317. https://doi.org/10.1016/j.vetmic.2014.08.011
Kato K (1966) Studies on calcium content in sea water: I. chelatometric determination of calcium in sea water. Bol Inst Oceanogr 15:25–28. https://doi.org/10.1590/S0373-55241966000100004
NurAsshifa MN, Zambry NS, Salwa MS, Yahya ARM (2017) The influence of agitation on oil substrate dispersion and oxygen transfer in Pseudomonas aeruginosa USM-AR2 fermentation producing rhamnolipid in a stirred tank bioreactor. 3 Biotech. 7:189. https://doi.org/10.1007/s13205-017-0828-0
Saejung C, Chaiyarat A, Sa-noamuang L (2018) Effects of algae, yeast and photosynthetic bacteria diets on survival and growth performance in the fairy shrimp, Streptocephalus sirindhornae (Branchiopoda, Anostraca). Crustaceana 91:1505–2152. https://doi.org/10.1163/15685403-00003847
Patthawaro S, Saejung C (2019) Production of single cell protein from manure as animal feed by using photosynthetic bacteria. MicrobiologyOpen. https://doi.org/10.1002/mbo3.913
Gao H, Khera E, Lee JK, Wen F (2016) Immobilization of multi-biocatalysts in alginate beads for cofactor regeneration and improved reusability. J Vis Exp. https://doi.org/10.3791/53944
Husken LE, Tramper J, Wijffels RH (1996) Growth and eruption of gel-entrapped microcolonies. In: Wijffels CRH, Buitelaar RM, Bucke C, Tramper J (eds) Immobilized cells: Basics and applications. Elsevier Science, Amsterdam, pp 336–340
Aarstad O, Strand BL, Klepp-Andersen LM, Skjak-Braek G (2013) Analysis of G-block distributions and their impact on gel properties of in vitro epimerized mannuronan. Biomacromolecules 14:3409–3416. https://doi.org/10.1021/bm400658k
Morris ER, Rees DA, Thom D, Boyd J (1978) Chiroptical and stoichiometric evidence of a specific, primary dimerisation process in alginate gelation. Carbohydr Res 66:145–154. https://doi.org/10.1016/S0008-6215(00)83247-4
Fang Y, Al-Assaf S, Phillips GO, Nishinari K, Funami T, Williams PA, Li L (2007) Multiple steps and critical behaviors of the binding of calcium to alginate. J Phys Chem B 111:2456–2462. https://doi.org/10.1021/jp0689870
Kim S, Jeong C, Cho S, Kim SB (2019) Effects of thermal treatment on the physical properties of edible calcium alginate gel beads: Response surface methodological approach. Foods 8:578. https://doi.org/10.3390/foods8110578
Teoh W, Kitayama K, Kobayashi M, Sato K (2009) Calcium alginate biopolymer for heavy metal wastewater treatment. J Ecotechnol Res 15:13–16. https://doi.org/10.11190/jer.14.46
Lin SF, Chen YC, Chen RN, Chen LC, Ho HO, Tsung YH, Sheu MT, Liu DZ (2016) Improving the stability of astaxanthin by microencapsulation in calcium alginate beads. PLoS ONE 11:e0153685. https://doi.org/10.1371/journal.pone.0153685
Jimenez-Diaz L, Caballero A, Segura A (2017) Pathways for the degradation of fatty acids in bacteria. In: Rojo F (ed) Aerobic utilization of hydrocarbons, oils and lipids. Handbook of hydrocarbon and lipid microbiology. Springer, Cham, pp 1–23
Juengert J, Bresan S, Jendrossek D (2018) Determination of polyhydroxybutyrate (PHB) content in Ralstonia eutropha using gas chromatography and nile red staining. Bio-Protocol 8:8. https://doi.org/10.21769/BioProtoc.2748
Mayeli N, Motamedi H, Heidarizadeh F (2015) Production of polyhydroxybutyrate by Bacillus axaraqunsis BIPC01 using petrochemical wastewater as carbon source. Braz Arch Biol Technol 58:643–650. https://doi.org/10.1590/S1516-8913201500048
Touloupakis E, Poloniataki EG, Casciana M, Ghanotakis DF, Carlozzi P (2021) Poly-β-hydroxybutyrate production by Rhodopseudomonas sp. grown in semi-continuous mode in a 4 L photobioreactor. Symmetry 13:1609. https://doi.org/10.3390/sym13091609
Padovani G, Emiliani G, Giovanelli A, Traversi ML, Carlozzi P (2018) Assessment of glycerol usage by five different purple non-sulfur bacterial strains for bioplastic production. J Environ Chem Eng 6:616–622. https://doi.org/10.1016/j.jece.2017.12.050
Acknowledgements
This work was supported by the Royal Golden Jubilee Scholarship (PHD/0156/2561), Thailand Science Research and Innovation (TSRI), and Research and Graduate Studies at Khon Kaen University.
Funding
This work was supported by the Royal Golden Jubilee Scholarship (PHD/0156/2561), Thailand Science Research and Innovation (TSRI), and Research and Graduate Studies at Khon Kaen University.
Author information
Authors and Affiliations
Contributions
A.C. and C.S. contributed to conceptualization; A.C. and C.S. contributed to methodology; A.C. and C.S. contributed to formal analysis and investigation; C.S. contributed to writing—original draft preparation, review, and editing; C.S. contributed to funding acquisition; C.S. provided the resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary material 1
Schematic diagram depicting the incorporation of Ca2+ into the beads after each removal cycle by immersing them in CaCl2 solution. (PNG 1166 kb)
Supplementary material 2
A Gaussian curve presenting the size distribution of alginate beads after each oil removal cycle. (PDF 166 kb)
Supplementary material 3
A Gaussian curve presenting the size distribution of alginate beads treated with CaCl2 after each oil removal cycle. (PDF 368 kb)

Supplementary material 4
Schematic diagram showing how immersion in CaCl2 solution improves bead stability after each removal cycle. (PNG 1556 kb)

Supplementary material 5
Fourier transform infrared (FTIR) spectra of polyhydroxybutyrate of the immobilized cells. (PNG 417 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaiyarat, A., Saejung, C. Recycling efficiency of Rhodopseudomonas faecalis PA2 immobilized in Ca-alginate for waste cooking oil removal. Biomass Conv. Bioref. 14, 10431–10442 (2024). https://doi.org/10.1007/s13399-022-03181-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03181-x