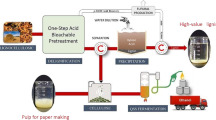
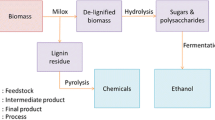
Abstract
The aim of this work was to develop a new green process for simultaneous depolymerization and valorization of lignin into high-value chemicals to meet the requirement of lignocellulosic biorefinery. A new Enzolv process for the delignification of the woody biomass of Melia dubia was developed using thermosolvent stable laccase (LccH) obtained from Hexagonia hirta MSF2. LccH showed enhanced initial activity of 127% and stability in the presence of ethanol, with 122% of its activity retained for 17 h. The process involved steam pretreatment of M. dubia biomass followed by laccase treatment (50 U.mL−1) in the presence of ethanol (2%) at 40 °C for 17 h. A maximum of 48% reduction in lignin and 52% availability of cellulose from woody biomass for further hydrolysis were obtained. Scanning electron microscopic analysis of the biomass revealed significant morphological and structural changes because of the Enzolv process. Significant lignin removal was observed by FT-IR, while an increase in cellulose content with a 20% increase in relative crystallinity compared to untreated biomass was observed by X-ray diffraction. This process also generated high-value chemicals such as benzaldehyde, isothiazole, propionic acid, benzene dicarboxylic acid, vanillin, and isovanillin during delignification.
Graphical abstract







Similar content being viewed by others
References
Luis Sanchez J, Hodge DB, Davis MF, et al (2019) Lignocellulosic biomass: understanding recalcitrance and predicting hydrolysis. Front Chem | www.frontiersin.org 7:874. https://doi.org/10.3389/fchem.2019.00874
Yoo CG, Meng X, Pu Y, Ragauskas AJ (2020) The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: a comprehensive review. Bioresour Technol 301:122784
Andreu G, Vidal T (2011) Effects of laccase-natural mediator systems on kenaf pulp. Bioresour Technol 102:5932–5937
Cao Y, Chen SS, Zhang S et al (2019) Advances in lignin valorization towards bio-based chemicals and fuels: lignin biorefinery. Bioresour Technol 291:121878
Hu J, Zhang Q, Lee D-J (2018) Kraft lignin biorefinery: a perspective. Bioresour Technol 247:1181–1183
Li X, Zheng Y (2020) Biotransformation of lignin: mechanisms, applications and future work. Biotechnol Prog 36:e2922
Ragauskas AJ, Beckham GT, Biddy MJ, Chandra R, Chen F, Davis MF, Wyman CE (2014) Lignin valorization: improving lignin processing in the biorefinery. Science 344(6185):1246843
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Zhao X, Cheng K, Liu D (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol Biotechnol 82:815–827
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci 7:163–173
Heap L, Green A, Brown D et al (2014) Role of laccase as an enzymatic pretreatment method to improve lignocellulosic saccharification. Catal Sci Technol 4:2251–2259
Andreu G, Vidal T (2013) Laccase from Pycnoporus cinnabarinus and phenolic compounds: can the efficiency of an enzyme mediator for delignifying kenaf pulp be predicted? Bioresour Technol 131:536–540
Mikulski D, Kłosowski G (2022) Delignification efficiency of various types of biomass using microwave-assisted hydrotropic pretreatment. Sci Rep 12:1–12. https://doi.org/10.1038/s41598-022-08717-9
Shin SK, Ko YJ, Hyeon JE, Han SO (2019) Studies of advanced lignin valorization based on various types of lignolytic enzymes and microbes. Bioresour Technol 289:121728
da Costa Lopes AM, Gomes JRB, Coutinho JAP, Silvestre AJD (2020) Novel insights into biomass delignification with acidic deep eutectic solvents: a mechanistic study of $β$-O-4 ether bond cleavage and the role of the halide counterion in the catalytic performance. Green Chem 22:2474–2487
Liu S, Liu H, Shen C, et al. (2021) Comparison of performances of different fungal laccases in delignification and detoxification of alkali-pretreated corncob for bioethanol production. J Ind Microbiol Biotechnol 48:kuab013
Kandasamy S, Muniraj IK, Purushothaman N et al (2016) High level secretion of laccase (LccH) from a newly isolated white-rot basidiomycete, Hexagonia hirta MSF2. Front Microbiol 7:707
Suman SK, Malhotra M, Kurmi AK et al (2022) Jute sticks biomass delignification through laccase-mediator system for enhanced saccharification and sustainable release of fermentable sugar. Chemosphere 286:131687. https://doi.org/10.1016/j.chemosphere.2021.131687
Thangavelu K, Desikan R, Taran OP, Uthandi S (2018) Delignification of corncob via combined hydrodynamic cavitation and enzymatic pretreatment: process optimization by response surface methodology. Biotechnol Biofuels 11:1–13
Sekar N, Andrey C, Uthandi S (2018) A two-step catalytic depolymerization of alkali treated Pennisetum glaucum L. and Melia dubia cav. into low molecular weight (LMW) aromatics. Madras Agric J 105:1
Sluiter A, Hames B, Ruiz R et al (2008) Determination of structural carbohydrates and lignin in biomass. Lab Anal Proced 1617:1–16
Suman SK, Khatri M, Dhawaria M et al (2018) Potential of Trametes maxima IIPLC-32 derived laccase for the detoxification of phenolic inhibitors in lignocellulosic biomass prehydrolysate. Int Biodeterior Biodegrad 133:1–8. https://doi.org/10.1016/j.ibiod.2018.05.009
Klibanov AM (1989) Enzymatic catalysis in anhydrous organic solvents. Trends Biochem Sci 14:141–144
Wu M-H, Lin M-C, Lee C-C et al (2019) Enhancement of laccase activity by pre-incubation with organic solvents. Sci Rep 9:1–11
Uthandi S, Saad B, Humbard MA, Maupin-Furlow JA (2010) LccA, an archaeal laccase secreted as a highly stable glycoprotein into the extracellular medium by Haloferax volcanii. Appl Environ Microbiol 76:733–743
Zumárraga M, Bulter T, Shleev S et al (2007) In vitro evolution of a fungal laccase in high concentrations of organic cosolvents. Chem Biol 14:1052–1064
Ziegler-Devin I, Chrusciel L, Brosse N (2021) Steam explosion pretreatment of lignocellulosic biomass: a mini-review of theorical and experimental approaches. Front Chem 9:1–7. https://doi.org/10.3389/fchem.2021.705358
El Hage R, Chrusciel L, Desharnais L, Brosse N (2010) Effect of autohydrolysis of Miscanthus x giganteus on lignin structure and organosolv delignification. Bioresour Technol 101:9321–9329
Moniruzzaman M, Ono T (2013) Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresour Technol 127:132–137
Rico A, Rencoret J, Del Río JC et al (2014) Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock. Biotechnol Biofuels 7:1–14
Rencoret J, Pereira A, del Río JC et al (2017) Delignification and saccharification enhancement of sugarcane byproducts by a laccase-based pretreatment. ACS Sustain Chem Eng 5:7145–7154
Banerjee R, Chintagunta AD, Ray S (2019) Laccase mediated delignification of pineapple leaf waste: an ecofriendly sustainable attempt towards valorization. BMC Chem 13:1–11
Sherpa KC, Ghangrekar MM, Banerjee R (2018) A green and sustainable approach on statistical optimization of laccase mediated delignification of sugarcane tops for enhanced saccharification. J Environ Manage 217:700–709
Soares B, da Costa Lopes AM, Silvestre AJD et al (2021) Wood delignification with aqueous solutions of deep eutectic solvents. Ind Crops Prod 160. https://doi.org/10.1016/j.indcrop.2020.113128
Shanmugam S, Hari A, Ulaganathan P et al (2018) Potential of biohydrogen generation using the delignified lignocellulosic biomass by a newly identified thermostable laccase from Trichoderma asperellum strain BPLMBT1. Int J Hydrogen Energy 43:3618–3628. https://doi.org/10.1016/j.ijhydene.2018.01.016
Ćilerdžić J, Stajić M, Vukojević J (2016) Degradation of wheat straw and oak sawdust by Ganoderma applanatum. Int Biodeterior Biodegrad 114:39–44. https://doi.org/10.1016/j.ibiod.2016.05.024
Adekunle AE, Zhang C, Guo C, Liu CZ (2017) Laccase production from Trametes versicolor in solid-state fermentation of steam-exploded pretreated cornstalk. Waste Biomass Valori 8:153–159. https://doi.org/10.1007/s12649-016-9562-9
Asgher M, Wahab A, Bilal M, Iqbal HMN (2018) Delignification of lignocellulose biomasses by alginate–chitosan immobilized laccase produced from Trametes versicolor IBL-04. Waste Biomass Valori 9:2071–2079. https://doi.org/10.1007/s12649-017-9991-0
Ravi K, García-Hidalgo J, Brink DP et al (2019) Physiological characterization and sequence analysis of a syringate-consuming Actinobacterium. Bioresour Technol 285:121327
Sainsbury PD, Hardiman EM, Ahmad M et al (2013) Breaking down lignin to high-value chemicals: the conversion of lignocellulose to vanillin in a gene deletion mutant of Rhodococcus jostii RHA1. ACS Chem Biol 8:2151–2156
García-Hidalgo J, Brink DP, Ravi K et al (2020) Vanillin production in Pseudomonas: whole-genome sequencing of Pseudomonas sp. strain 9.1 and reannotation of Pseudomonas putida CalA as a vanillin reductase. Appl Environ Microbiol 86:e02442-e2519
Li F, Ma F, Zhao H et al (2019) A lytic polysaccharide monooxygenase from a white-rot fungus drives the degradation of lignin by a versatile peroxidase. Appl Environ Microbiol 85:e02803-e2818
Westereng B, Cannella D, Agger JW et al (2015) Enzymatic cellulose oxidation is linked to lignin by long-range electron transfer. Sci Rep 5:1–9
Abraham RE, Vongsvivut J, Barrow CJ, Puri M (2016) Understanding physicochemical changes in pretreated and enzyme hydrolysed hemp (Cannabis sativa) biomass for biorefinery development. Biomass Convers biorefinery 6:127–138
Corrales RCNR, Mendes FMT, Perrone CC et al (2012) Structural evaluation of sugar cane bagasse steam pretreated in the presence of CO 2 and SO 2. Biotechnol Biofuels 5:1–8
Boeriu CG, Bravo D, Gosselink RJA, van Dam JEG (2004) Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind Crops Prod 20:205–218
Liu X, Li T, Wu S et al (2020) Structural characterization and comparison of enzymatic and deep eutectic solvents isolated lignin from various green processes: toward lignin valorization. Bioresour Technol 310:123460
Mukherjee A, Banerjee S, Halder G (2018) Parametric optimization of delignification of rice straw through central composite design approach towards application in grafting. J Adv Res 14:11–23
Funding
The authors are grateful to the Department of Biotechnology, Government of India, for financial support through a competitive grant No/BT/PR/8625/PBD/26/27/2007 and SERB for financial support through EEQ No. EEQ/2020/000583 given to SU.
Author information
Authors and Affiliations
Contributions
Conceptualization: US. Data curation and formal analysis: IKM, PVA. Funding acquisition: US. Methodology: US, KTP, IKM, PVA. Project administration: US. Writing original draft: IKM, US, and PVA. Writing-review and editing: US.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muniraj, I.K., Anbu, P.V., Parthiban, K.T. et al. A new Enzolv process for simultaneous delignification and lignin-derived chemical production from the woody biomass of Melia dubia. Biomass Conv. Bioref. 13, 14557–14571 (2023). https://doi.org/10.1007/s13399-022-03084-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03084-x