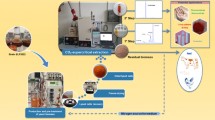
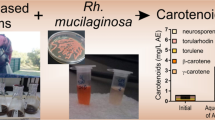
Abstract
Over the last few years, there has been an impetuous search for natural colorant to be applied in various industries, such as cosmetics, food, and textile. This is largely driven by an increasing consumer demand for formulated ingredient products that are not only considered safer than their synthetic counterparts, but also present medicinal health benefits. However, there are a limited number of commercially available soluble organic pigments. Rhodotorula glutinis yeast can be used for the biodegradation of phenols rich olive mill wastewater, which is an abundant and jeopardous polluting waste, while simultaneously producing multiple high-added value compounds, such as the natural colorant β-carotene. Herein, the protocols were firstly optimized by screening 10 different solvents while evaluating their aptitude to dissolve pure β-carotene. The results followed the trend: water ≈ methanol < ethanol < acetonitrile < acetone < dichloromethane < diethyl ether < heptane < ethyl acetate < benzene. However, due to the high toxicity of benzene and the carotenoid precipitation by heptane, ethyl acetate, diethyl ether, and dichloromethane were selected as the most promising solvents. Thus, an integrated process for the extraction, isolation, and concentration of β-carotene was proposed from Rhodotorula glutinis, cultivated in (processing) effluent. The extraction results followed the same tendency as for pure carotenoid: dichloromethane (13 ± 1 μg g–1 yeast) < diethyl ether (15 ± 2 μg g–1 yeast) < ethyl acetate (21 ± 2 μg g–1 yeast). Regarding liquid–liquid extraction, the maximum extraction efficiency was also achieved with ethyl acetate, i.e., 100 ± 28%. Overall, these results show promising findings for a cascade biorefinery downstream process, as this not only potentially reduces the burden on the environment, but also adds value to primary economic activity.
Graphical abstract





Similar content being viewed by others
Data availability statement
Authors can confirm that all relevant data are included in the article and/or its supplementary information files.
References
Aggoun M, Arhab R, Cornu A et al (2016) Olive mill wastewater microconstituents composition according to olive variety and extraction process. Food Chem 209:72–80. https://doi.org/10.1016/j.foodchem.2016.04.034
Khdair A Abu-Rumman G (2020) sustainable environmental management and valorization options for olive mill byproducts in the Middle East and North Africa (MENA) region Processes 8. https://doi.org/10.3390/pr8060671
Foti P Romeo FV Russo N et al (2021) Olive mill wastewater as renewable raw materials to generate high added-value ingredients for agro-food industries Appl Sci 11. https://doi.org/10.3390/app11167511
Zimmerman JB, Anastas PT, Erythropel HC, Leitner W (2020) Designing for a green chemistry future. Science 367:397–400. https://doi.org/10.1126/science.aay3060
Karakaya A, Laleli Y, Takaç S (2012) Development of process conditions for biodegradation of raw olive mill wastewater by Rhodotorula glutinis. Int Biodeterior Biodegradation 75:75–82. https://doi.org/10.1016/j.ibiod.2012.09.005
Karakaya A Bozkoyunlu G Laleli Y Takaç S (2014) Development of pH adjustment-based operational strategy to increase total phenol removal rate in biodegradation of olive mill wastewater by Rhodotorula glutinis Desalin Water Treat. https://doi.org/10.1080/19443994.2013.823357
Schneider T, Graeff-Hönninger S, French WT et al (2013) Lipid and carotenoid production by oleaginous red yeast Rhodotorula glutinis cultivated on brewery effluents. Energy 61:34–43. https://doi.org/10.1016/j.energy.2012.12.026
Park PK, Kim EY, Chu KH (2007) Chemical disruption of yeast cells for the isolation of carotenoid pigments. Sep Purif Technol 53:148–152. https://doi.org/10.1016/j.seppur.2006.06.026
Saha S, Walia S, Kundu A et al (2015) Optimal extraction and fingerprinting of carotenoids by accelerated solvent extraction and liquid chromatography with tandem mass spectrometry. Food Chem 177:369–375. https://doi.org/10.1016/j.foodchem.2015.01.039
Amorim-Carrilho KT, Cepeda A, Fente C, Regal P (2014) Review of methods for analysis of carotenoids. Trac Trends Anal Chem 56:49–73. https://doi.org/10.1016/j.trac.2013.12.011
Hernández-Almanza A, Montanez JC, Aguilar-González MA et al (2014) Rhodotorula glutinis as source of pigments and metabolites for food industry. Food Biosci 5:64–72. https://doi.org/10.1016/j.fbio.2013.11.007
de Souza Mesquita LM, Martins M, Pisani LP et al (2021) Insights on the use of alternative solvents and technologies to recover bio-based food pigments. Compr Rev Food Sci Food Saf 20:787–818. https://doi.org/10.1111/1541-4337.12685
Mussagy CU Khan S Kot AM (2021) Current developments on the application of microbial carotenoids as an alternative to synthetic pigments. Crit Rev Food Sci Nutr 0:1–15. https://doi.org/10.1080/10408398.2021.1908222
Michelon M, de Matos de Borba T, da Silva Rafael R, et al (2012) Extraction of carotenoids from Phaffia rhodozyma: a comparison between different techniques of cell disruption. Food Sci Biotechnol 21:1–8. https://doi.org/10.1007/s10068-012-0001-9
Bogacz-Radomska L, Harasym J (2018) β-Carotene—properties and production methods. Food Qual Saf 2:69–74. https://doi.org/10.1093/fqsafe/fyy004
Marova I, Carnecka M, Halienova A et al (2012) Use of several waste substrates for carotenoid-rich yeast biomass production. J Environ Manage 95:S338–S342. https://doi.org/10.1016/j.jenvman.2011.06.018
Tinoi J, Rakariyatham N, Deming RL (2005) Simplex optimization of carotenoid production by Rhodotorula glutinis using hydrolyzed mung bean waste flour as substrate. Process Biochem 40:2551–2557. https://doi.org/10.1016/j.procbio.2004.11.005
Mussagy C Coutinho J Pereira J (2021) Production of carotenoids by yeast Rhodotorula glutinis CCT-2186 and their extraction using alternative solvents
Mussagy C Gonzalez-Miquel M Ebinuma V Pereira J (2022) Microbial torularhodin - a comprehensive review Crit Rev Biotechnol 1. https://doi.org/10.1080/07388551.2022.2041540
Mussagy C Ebinuma V Carvalho P et al (2020) Integrative platform for the selective recovery of intracellular carotenoids and lipids from Rhodotorula glutinis CCT-2186 yeast using mixtures of bio-based solvents † Green Chem 22. https://doi.org/10.1039/d0gc02992k
Saini RK, Keum Y-S (2018) Carotenoid extraction methods: a review of recent developments. Food Chem 240:90–103. https://doi.org/10.1016/j.foodchem.2017.07.099
Aksu Z, Eren AT (2007) Production of carotenoids by the isolated yeast of Rhodotorula glutinis. Biochem Eng J 35:107–113. https://doi.org/10.1016/j.bej.2007.01.004
Clarke KG (2013) 11 - Downstream processing. In: Clarke KG (ed) Bioprocess Engineering. Woodhead Publishing, pp 209–234
Mezzomo N, Maestri B, dos Santos RL et al (2011) Pink shrimp (P. brasiliensis and P. paulensis) residue: Influence of extraction method on carotenoid concentration. Talanta 85:1383–1391. https://doi.org/10.1016/j.talanta.2011.06.018
Minguez-Mosquera MI, Hornero-Mendez D (1993) Separation and quantification of the carotenoid pigments in red peppers (Capsicum annuum L.), paprika, and oleoresin by reversed-phase HPLC. J Agric Food Chem 41:1616–1620. https://doi.org/10.1021/jf00034a018
Alder CM, Hayler JD, Henderson RK et al (2016) Updating and further expanding GSK{’}s solvent sustainability guide. Green Chem 18:3879–3890. https://doi.org/10.1039/C6GC00611F
Byrne FP Jin S Paggiola G et al (2016) Tools and techniques for solvent selection: green solvent selection guides. Sustain Chem Process 4:7. https://doi.org/10.1186/s40508-016-0051-z
Dirgha Raj Joshi Nisha Adhikari (2019) An Overview on Common Organic Solvents and Their Toxicity. J Pharm Res Int 28:1–18. https://doi.org/10.9734/jpri/2019/v28i330203
Rivera S, Canela R (2012) Influence of sample processing on the analysis of carotenoids in maize. Molecules 17:11255–11268. https://doi.org/10.3390/molecules170911255
Craft NE, Soares JH (1992) Relative solubility, stability, and absorptivity of lutein and.beta.-carotene in organic solvents. J Agric Food Chem 40:431–434. https://doi.org/10.1021/jf00015a013
Prat D, Pardigon O, Flemming H-W et al (2013) Sanofi’s solvent selection guide: a step toward more sustainable processes. Org Process Res Dev 17:1517–1525. https://doi.org/10.1021/op4002565
Shakeel F, Haq N, Alshehri S et al (2018) Solubility, thermodynamic properties and solute-solvent molecular interactions of luteolin in various pure solvents. J Mol Liq 255:43–50. https://doi.org/10.1016/j.molliq.2018.01.155
Simpson KL, Nakayama TOM, Chichester CO (1964) Biosynthesis of yeast carotenoids. J Bacteriol 88:1688–1694. https://doi.org/10.1128/jb.88.6.1688-1694.1964
Moliné M, Libkind D, van Broock M (2012) Production of torularhodin, torulene, and $β$-carotene by rhodotorula yeasts. In: Barredo J-L (ed) Microbial Carotenoids From Fungi: Methods and Protocols. Humana Press, Totowa, NJ, pp 275–283
Moliné M, Flores MR, Libkind D et al (2010) Photoprotection by carotenoid pigments in the yeast Rhodotorula mucilaginosa: the role of torularhodin. Photochem Photobiol Sci 9:1145–1151. https://doi.org/10.1039/c0pp00009d
Sharma R, Ghoshal G (2019) Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: a statistical approach. Biotechnol Reports 25:e00407. https://doi.org/10.1016/j.btre.2019.e00407
Louli V, Ragoussis N, Magoulas K (2004) Recovery of phenolic antioxidants from wine industry by-products. Bioresour Technol 92:201–208. https://doi.org/10.1016/j.biortech.2003.06.002
Prommuak C, De-Eknamkul W, Shotipruk A (2008) Extraction of flavonoids and carotenoids from Thai silk waste and antioxidant activity of extracts. Sep Purif Technol 62:444–448. https://doi.org/10.1016/j.seppur.2008.02.020
Funding
This research was funded by the ERA CoBioTech within the project Rhodolive and Slovenian Research Agency within the projects P2–0152, J2-1723, J2-2492 and NC-0013.
Author information
Authors and Affiliations
Contributions
Lucija Hladnik, data curation; formal analysis; investigation; methodology; resources; validation; visualization; roles/writing—original draft; writing—review and editing. Filipa A. Vicente, conceptualization; supervision; formal analysis; writing—review and editing. Miha Grilc, writing—review and editing. Blaž Likozar, conceptualization, funding acquisition, project administration, supervision, writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hladnik, L., Vicente, F.A., Grilc, M. et al. β-Carotene production and extraction: a case study of olive mill wastewater bioremediation by Rhodotorula glutinis with simultaneous carotenoid production. Biomass Conv. Bioref. 14, 8459–8467 (2024). https://doi.org/10.1007/s13399-022-03081-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03081-0