Abstract
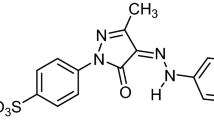
This study focuses on the utilization of Fe3O4-functionalized rice straw biochar composite for the augmented activation of sulphate radicals at high temperature for the removal of Remazol Brilliant Orange 3R (RBO-3R). The process parameters were optimized using factorial modelling and were mathematically evaluated using Statistical Analysis System (SAS) programming and canonical-ridge analysis. The following optimized conditions for maximum decolourization of 365.78 mg/L RBO-3R dye were found: temperature of 80 °C, persulphate concentration of 5.22 mM, dye concentration of 500 mg/L and time of 30 min. A novel rice straw–based biochar (RC-BC) was then prepared through pyrolysis and impregnated with iron to synthesize ferrous-loaded biochar (Fe-BC). The surface morphology of Fe biochar was characterized using scanning electron microscopy-energy-dispersive X-ray (SEM–EDX), and their crystal structures were analysed using X-ray diffraction (XRD) analysis. The kinetic rate constants for the thermal-activated persulphate and Fe-BC and Fe-BC-incorporated thermal-activated persulphate system were 0.0475 min−1, 0.0068 min−1 and 0.0724 min−1, respectively. The toxicity analysis of the degraded metabolites of gas chromatography mass spectrometry (GC–MS) using ProTox and EPI Suite models showed that the products had lower LD50 values, indicating their safer discharge into the environment. Despite this application, this functionalized rice straw biochar can be applied as soil amendments for heavy metal stabilisation, supports for enzyme immobilization, photocatalysts and adsorbents for removing environmental pollutants.







Similar content being viewed by others
Data availability
E-supplementary data of this manuscript can be found in the online version of the paper.
References
Lellis B, Fávaro-Polonio CZ, Pamphile JA, Polonio JC (2019) Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov 3:275–290. https://doi.org/10.1016/j.biori.2019.09.001
Wang C, Sun R, Huang R (2021) Highly dispersed iron-doped biochar derived from sawdust for Fenton-like degradation of toxic dyes. J Clean Prod 297:126681. https://doi.org/10.1016/j.jclepro.2021.126681
Kishor R, Purchase D, Saratale GD et al (2021) Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J Environ Chem Eng 9.https://doi.org/10.1016/j.jece.2020.105012
Pandi A, Marichetti Kuppuswami G, Numbi Ramudu K, Palanivel S (2019) A sustainable approach for degradation of leather dyes by a new fungal laccase. J Clean Prod 211:590–597. https://doi.org/10.1016/j.jclepro.2018.11.048
Muniyasamy A, Sivaporul G, Gopinath A et al (2020) Process development for the degradation of textile azo dyes (mono-, di-, poly-) by advanced oxidation process - ozonation: experimental & partial derivative modelling approach. J Environ Manage 265.https://doi.org/10.1016/j.jenvman.2020.110397
Maas R, Chaudhari S (2005) Adsorption and biological decolourization of azo dye Reactive Red 2 in semicontinuous anaerobic reactors. Process Biochem 40:699–705. https://doi.org/10.1016/j.procbio.2004.01.038
Mbarki F, Kesraoui A, Seffen M, Ayrault P (2018) Kinetic, thermodynamic, and adsorption behavior of cationic and anionic dyes onto corn stigmata: nonlinear and stochastic analyses. Water Air Soil Pollut 229.https://doi.org/10.1007/s11270-018-3749-6
Paba GM, Ávila RB, Baldiris DB (2021) Application of environmental bacteria as potential methods of azo dye degradation systems. Glob J Environ Sci Manag 7:131–154. https://doi.org/10.22034/gjesm.2021.01.10
Martínez-López S, Lucas-Abellán C, Serrano-Martínez A et al (2019) Pulsed light for a cleaner dyeing industry: azo dye degradation by an advanced oxidation process driven by pulsed light. J Clean Prod 217:757–766. https://doi.org/10.1016/j.jclepro.2019.01.230
Guerra-Rodríguez S, Rodríguez E, Singh DN, Rodríguez-Chueca J (2018) Assessment of sulfate radical-based advanced oxidation processes for water and wastewater treatment: a review. Water (Switzerland) 10.https://doi.org/10.3390/w10121828
Yabalak E (2021) Treatment of agrochemical wastewater by thermally activated persulfate oxidation method: Evaluation of energy and reagent consumption. J Environ Chem Eng 9.https://doi.org/10.1016/j.jece.2021.105201
Rodríguez-Carmona E, Manresa A, Bastida J (2013) Application of experimental design and canonical analysis of response surfaces to the optimization of poly(3-hydroxyalkanoates) production by Pseudomonas aeruginosa 42A2. Chem Biochem Eng Q 27:457–465
Rajendran HK, Deen Fakrudeen MA, Chandrasekar R et al (2021) Electrocatalytic removal of fluroquinolones from simulated pharmaceutical effluent: chemometric analysis, chemical blueprint of electrodes and generated sludge. Environ Res 195.https://doi.org/10.1016/j.envres.2021.110844
Balamurugan N, Annamalai K, Gandhi Sethuraman MI et al (2021) Chemometric approach towards optimization on the radiolytic removal of micropollutants: equation based modelling with canonical and ridge analysis. Chemom Intell Lab Syst 208.https://doi.org/10.1016/j.chemolab.2020.104222
Hattab MW (2018) On the use of data transformation in response surface methodology. Qual Reliab Eng Int 34:1185–1194. https://doi.org/10.1002/qre.2317
Miller DM (1984) Reducing transformation bias in curve fitting. Am Stat 38:124. https://doi.org/10.2307/2683247
Lin BT, Der JM, Chou JH (2007) Using response surface methodology with response transformation in optimizing plasma spraying coatings. Int J Adv Manuf Technol 34:307–315. https://doi.org/10.1007/s00170-006-0599-y
Huang H, Guo T, Wang K et al (2021) Efficient activation of persulfate by a magnetic recyclable rape straw biochar catalyst for the degradation of tetracycline hydrochloride in water. Sci Total Environ 758.https://doi.org/10.1016/j.scitotenv.2020.143957
Bibi A, Zhu H-x, Mahmood Q et al (2020) Efficient bacterial isolate from roots of cactus degrading Reactive Black 5. Environ Technol Innov 20.https://doi.org/10.1016/j.eti.2020.101082
Balakrishnan A, Kanchinadham SBK, Kalyanaraman C (2020) Assessment on biodegradability prediction of tannery wastewater using EPI Suite BIOWIN model. Environ Monit Assess 192.https://doi.org/10.1007/s10661-020-08661-z
Ike IA, Orbell JD, Duke M (2018) Feasibility, mechanisms, and optimisation of organic pollutant degradation by thermally activated persulphate. Chem Eng Res Des 136:304–314. https://doi.org/10.1016/j.cherd.2018.05.041
Ghauch A, Tuqan AM (2012) Oxidation of bisoprolol in heated persulfate/H2O systems: kinetics and products. Chem Eng J 183:162–171. https://doi.org/10.1016/j.cej.2011.12.048
Ma J, Li H, Chi L et al (2017) Changes in activation energy and kinetics of heat-activated persulfate oxidation of phenol in response to changes in pH and temperature. Chemosphere 189:86–93. https://doi.org/10.1016/j.chemosphere.2017.09.051
Ahmadi S, Igwegbe CA, Rahdar S (2019) The application of thermally activated persulfate for degradation of Acid Blue 92 in aqueous solution. Int J Ind Chem 10:249–260. https://doi.org/10.1007/s40090-019-0188-1
Weng CH, Tsai KL (2016) Ultrasound and heat enhanced persulfate oxidation activated with Fe0 aggregate for the decolorization of C.I. Direct Red 23. Ultrason Sonochem 29:11–18. https://doi.org/10.1016/j.ultsonch.2015.08.012
Bezerra MA, Santelli RE, Oliveira EP et al (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Aydar AY (2018) Utilization of response surface methodology in optimization of extraction of plant materials. Statistical approaches with emphasis on design of experiments applied to chemical processes. 157–169. https://doi.org/10.5772/intechopen.73690
Tan Z, Wang Y, Kasiulienė A et al (2017) Cadmium removal potential by rice straw-derived magnetic biochar. Clean Technol Environ Policy 19:761–774. https://doi.org/10.1007/s10098-016-1264-2
Jindo K, Mizumoto H, Sawada Y et al (2014) Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 11:6613–6621. https://doi.org/10.5194/bg-11-6613-2014
Hu X, Ding Z, Zimmerman AR et al (2015) Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res 68:206–216. https://doi.org/10.1016/j.watres.2014.10.009
Author information
Authors and Affiliations
Contributions
Juliana John: data curation and writing; R. Gandhimathi: review and editing, and supervision; Mika Sillanpaa: review and editing; Padmanaban Velayudhaperumal Chellam: conceptualization, review and editing, and supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
John, J., Gandhimathi, R., Sillanpää, M. et al. Fe3O4-functionalised biochar for persulphate systems towards the removal of Remazol Brilliant Orange 3R: machine learning–based approach and toxicity analysis. Biomass Conv. Bioref. 14, 10319–10334 (2024). https://doi.org/10.1007/s13399-022-03056-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03056-1