Abstract
Yeasts have received significant attention in recent years as major sources of value-added metabolites endowed with various natural biological activities. Among the yeasts studied until now, the so-called red yeasts have a great potential in microbial lipids and carotenoids production serving as precursor for biofuels, oleo-chemicals, and food additives. In this work, biodiesel-derived crude glycerol was used as feedstock for concomitant valuable metabolites production by the oleaginous yeast Rhodotorula babjevae Y-SL7. Under specific conditions, this strain has been shown to accumulate a high intracellular content of microbial oil (> 40%) and to secrete a mixture of polyol esters of fatty acids (PEFA). Using fed-batch fermentation, the appropriate culture conditions were established for maximum lipids and carotenoids production. The characterization of extracted carotenoids reveals the presence of two major compounds, the torularhodin (63.7%) and torulene (36.3%) and their related antimicrobial and antioxidant activities were investigated. Moreover, secreted PEFAs showed therapeutically promising cytotoxic effect against cancer cells and their synergistic action with commercial drug was also established. On the other hand, flow cytometry analysis showed that culture on crude glycerol increases cells membrane permeability and further enhances metabolites recovery. This can facilitate downstream processing and therefore increase the profitability of the production system. Indeed, the present study opens new perspectives for multifunctional metabolites production using cheap industrial by-product through completely eco-friendly processes




Similar content being viewed by others
References
Pacwa-Plociniczak M, Plaza GA, Piotrowska-Seget Z, Cameotra SS (2011) Environmental applications of biosurfactants: recent advances. Int J Mol Sci 12:633–654
Garay LA, Sitepu IR, Cajka T, Cathcart E, Fiehn O, German JB, Block DE, Boundy-Mills KL (2017) Simultaneous production of intracellular triacylglycerols and extracellular polyol esters of fatty acids by RhodotorulababjevaeandRhodotorulaaff. paludigena.J Ind Microbiol Biotechnol 44:1397–1413
Clauser NM, González G, Mendieta CM, Kruyeniski J, Area MC, Vallejos ME (2021) Biomass waste as sustainable raw material for energy and fuels. Sustainability 13:794
Lyman M, Urbin S, Strout C, Rubinfeld B (2019) The oleaginous red yeast Rhodotorula/Rhodosporidium: a factory for industrial bioproducts. In: Yeasts in biotechnology. IntechOpen https://doi.org/10.5772/intechopen.84129.
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86(11):807–815
Suleeporn K, Benjamas C (2013) Enhancing lipid production from crude glycerol by newly isolated oleaginous yeasts: strain selection, process optimization, and fed-batch strategy. Bioenerg Res 6(1):300–310
Beopoulos A, Nicaud JM (2012) Yeast: a new oil producer. OCL 19(1):22–28
Zhang F, Rodriguez S, Keasling JD (2011) Metabolic engineering of microbial pathways for advanced biofuels production. Curr Opin Biotechnol 22(6):775–783
Ghanavati H, Nahvi I, Karimi K (2015) Organic fraction of municipal solid waste asa suitable feedstock for the production of lipid by oleaginous yeast Cryptococcus aerius. Waste Manag 38:141–148
Ayadi I, Belghith H, Gargouri A, Guerfali M (2019) Utilization of wheat bran acid hydrolysate by Rhodotorula mucilaginosa Y-MG1 for microbial lipid production as feedstock for biodiesel synthesis. Biomed Res Int 2019:1–11
Liu Z, Natalizio F, Dragone G, Mussatto SI (2021) Maximizing the simultaneous production of lipids and carotenoids by Rhodosporidium toruloides from wheat straw hydrolysate and perspectives for large-scale implementation. Bioresour Technol 340:125598
Chmielarz M, Blomqvist J, Sampels S, Sandgren M, Passoth V (2021) Microbial lipid production from crude glycerol and hemicellulosic hydrolysate with oleaginous yeasts. Biotechnol Biofuels 14:65
Schneider T, Graeff-Hönninger S, French WT, Hernandez R, Merkt N, Claupein W, Hetrick M, Pham P (2013) Lipid and carotenoid production by oleaginous red yeast Rhodotorula glutinis cultivated on brewery effluents. Energy 61:34–43
Arous F, Atitallah IB, Nasri M, Mechichi T (2017) A sustainable use of low-cost raw substrates for biodiesel production by the oleaginous yeastWickerhamomycesanomalus.3 Biotech 7:268
Manowattana A, Techapun C, Watanabe M, Chaiyaso T (2018) Bioconversion of biodiesel-derived crude glycerol into lipids and carotenoids by an oleaginous red yeast Sporidiobolus pararoseus KM281507 in an airlift bioreactor. J biosci bioeng 125:59–66
Garlapati VK, Shankar U, Budhiraja A (2016) Bioconversion technologies of crude glycerol to value added industrial products. Biotechnol Rep 9:9–14
Saifuddin NM, Hussein R, Ong MY (2018) Sustainability of biodiesel production in Malaysia by production of bio-oil from crude glycerol using microwave pyrolysis: a review. Green Chem Lett Rev 11(2):135–157
Uprety BK, Dalli SS, Rakshit SK (2017) Bioconversion of crude glycerol to microbial lipid using a robust oleaginous yeast Rhodosporidium toruloides ATCC 10788 capable of growing in the presence of impurities. Energy Convers Manag 135:117–128
Buedenbender L, Kumar A, Blümel M, Kempken F, Tasdemir D (2021) Genomics- and metabolomics-based investigation of the deep-sea sediment-derived yeast, Rhodotorula mucilaginosa 50-3-19/20B. Mar Drugs 19:14
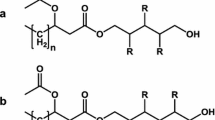
Guerfali M, Ayadi I, Mohamed N, Ayadi W, Belghith H, Bronze MR, Ribeiro MH, Gargouri A (2019) Triacylglycerols accumulation and glycolipids secretion by the oleaginous yeast Rhodotorula babjevae Y-SL7: structural identification and biotechnological applications. Bioresour Technol 273:326–334
Cortés-Sánchez AJ, Hernandez-Sanchez H, Jaramillo-Flores ME (2013) Biological activity of glycolipids produced by microorganisms: new trends and possible therapeutic alternatives. Microbiol Res 168:22–32
Lyman M, Rubinfeld B, Leif R, Mulcahy H, Dugan L, Souza B (2018) Rhodotorula taiwanensis MD1149 produces hypoacetylated PEFA compounds with increased surface activity compared to Rhodotorula babjevae MD1169. PLoS One 13(1):e0190373
Abdel-Mawgoud AM, Lépine F, Déziel E (2010) Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86:1323–1336
Van Bogaert IN, Saerens K, De Muynck C, Develter D, Soetaer W, Vandamme EJ (2007) Microbial production and application of sophorolipids. Appl Microbiol Biotechnol 76:23–34
Mata-Gómez LC, Montañez JC, Méndez-Zavala A, Aguilar CN (2014) Biotechnological production of carotenoids by yeasts: an overview. Microb Cell Fact 13:1–11
Tang W, Wang Y, Zhang J, Cai Y, He Z (2019) Biosynthetic pathway of carotenoids in Rhodotorula and strategies for enhanced their production. J Microbiol Biotechnol 29(4):507–517
Guerfali M, Ayadi I, Sassi HE, Belhassen A, Gargouri A, Belghith H (2020) Biodiesel-derived crude glycerol as alternative feedstock for single cell oil production by the oleaginous yeast Candida viswanathii Y-E4. Ind Crops Prod 145:112103
Guerfali M, Ayadi I, Belhassen A, Gargouri A, Belghith H (2018) Single cell oil production by Trichosporon cutaneum and lignocellulosic residues bioconversion for biodiesel synthesis. Process Saf Environ Prot 113:292–304
Ayadi I, Belghith H, Gargouri A, Guerfali M (2018) Screening of new oleaginous yeasts for single cell oil production, hydrolytic potential exploitation and agro-industrial by-products valorization. Process Saf Environ Prot 119:104–114
Ayadi I, Kamoun O, Trigui-Lahiani H, Hdiji A, Gargouri A, Belghith H, Guerfali M (2016) Single cell oil production from a newly isolated Candida viswanathii Y-E4 and agro-industrial by-products valorization. J Ind Microbiol Biotechnol 43(7):901–914
Dey P, Maiti MK (2013) Molecular characterization of a novel isolate of Candida tropicalis for enhanced lipid production. Appl Microbiol 114(5):1357–1368
Taskin M, Sisman T, Erdal S, Basaran EK (2011) Use of waste chicken feathers as peptone for production of carotenoids in submerged culture of Rhodotorula glutinis MT-5. Eur Food Res Technol 233:657–665
Miller GL (1959) Use of dinitrosalicylic acid reagent for thedetermination of reducing sugar. Anal Chem 31(3):426–428
McKenzie H, Wallace HS (1954) The Kjeldahl determination of nitrogen: a critical study of digestion conditions-temperature, catalyst, and oxidizing agent. Aust J Chem 7(1):55–70
Elfeky N, Elmahmoudy M, Zhang Y, Guo J, Bao Y (2019) Lipid and carotenoid production by Rhodotorula glutinis with a combined cultivation mode of nitrogen, sulfur, and aluminium stress. Appl Sci 9:2444
Yehia M, Al-Olayan EM, Elkhadragy MF, Khalaf-Allah AM, El-Shimi NM (2013) Improvement of carotenoid pigments produced by Rhodotorula glutinis Hany. Life Sci J 10(4):386–400
Falcioni T, Papa S, Gasol JM (2008) Evaluating the flow-cytometric nucleic acid double-staining protocol in realistic situations of planktonic bacterial death. Appl Environ Microbiol 74(6):1767–1779
Gulluce M, Sahin F, Sokmen M, Ozer H, Daferera D, Sokmen A, Polissiou M, Adiguzel H, Ozkan H (2007) Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L ssp longifolia. Food chem 103(4):1449–1456
Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9–10):1231–1237
Diamantopoulou P Filippousi R Antoniou D Varfi E Xenopoulos E Sarris D Papanikolaou S (2020) Production of added-value microbial metabolites during growth of yeast strains on media composed of biodiesel-derived crude glycerol and glycerol/xylose blends. FEMS Microbiol Lett 367: fnaa063
Pereira RN, da Silveira JM, Burkert JFM, Ores JDC, Burkert CAV (2019) Simultaneous lipid and carotenoids production by stepwise fed-batch cultivation of Rhodotorula mucilaginosa with crude glycerol. Braz J Chem Eng 36(3):1099
Saenge C, Cherisilp B, Suksaroge TT, Bourtoom T (2011) Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem 46:210–218
Yousuf A, Sannino F, Addorisio V, Pirozzi D (2010) Microbial conversion of olive mill waste waters into lipids suitable for biodiesel production. J Agric Food Chem 58:8630–8635
Ghilardi C, Negrete PS, Carelli AA, Borroni V (2020) Evaluation of olive mill waste as substrate for carotenoid production by Rhodotorula mucilaginosa. Bioresour Bioprocess 7:52
Dourou M, Kancelista A, Juszczyk P, Sarris D, Bellou S, Triantaphyllidou I, Rywinska A, Papanikolaou S, Aggelis G (2016) Bioconversion of olive mill wastewater into high-added value products. J Clean Prod 139:957–969
Sarris D, Stoforos NG, Mallouchos A, Kookos IK, Koutinas AA, Aggelis G, Papanikolaou S (2017) Production of added-value metabolites by Yarrowia lipolytica growing in olive mill wastewater-based media under aseptic and non-aseptic conditions. Eng Life Sci 17:695–709
Valdés G, Mendonça RT, Aggelis G (2020) Lignocellulosic biomass as a substrate for oleaginous microorganisms: a review. Appl Sci 10:7698
Zipper H, Brunner H, Bernhagen J, Vitzthum F (2004) Investigations on DNA intercalationand surface binding by SYBR Green I, its structure determination and methodologi-cal implications. Nucleic Acids Res 32:103
Davey HM, Hexley P (2011) Red but not dead? Membranes of stressed Saccharomyces cerevisiae are permeable to propidium iodide. Environ Microbiol 13(1):163–171
Kosa G, Shapaval V, Kohler A, Zimmermann B (2017) FTIR spectroscopy as a unified method for simultaneous analysis of intra- and extracellular metabolites in high-throughput screening of microbial bioprocesses. Microb Cell Fact 16:195
Shapaval V, Brandenburg J, Blomqvist J, Tafintseva V, Passoth V, Sandgren M, Kohler A (2019) Biochemical profiling, prediction of total lipid content and fatty acid profile in oleaginous yeasts by FTIR spectroscopy. Biotechnol Biofuels 12:140
Sáez-Bastante J, Pinzi S, Reyero I, Priego-Capote F, Luque de Castro MD, Dorado MP (2014) Biodiesel synthesis from saturated and unsaturated oils assisted by the combination of ultrasound, agitation and heating. Fuel 131:6–16
Pinzi S, Mata-Granados JM, Lopez-Gimenez FJ, Luque de Castro MD, Dorado MP (2011) Influence of vegetable oils fatty-acid composition on biodiesel optimization. Bioresour Technol 102(2):1059–1065
Sharma R, Ghoshal G (2021) Characterization and cytotoxic activity of pigment extracted from Rhodotorula mucilaginosa to assess its potential as biofunctional additive in confectionary products. J Food Sci Technol 58(7):2688–2698
Park PK, Kim EY, Chu KH (2007) Chemical disruption of yeast cells for the isolation of carotenoid pigments. Sep Purif Technol 53:148–152
Kot AM, Blazejak S, Gientka I, Kieliszek M, Brys J (2018) Torulene and torularhodin: “new” fungal carotenoids for industry. Microb Cell Fact 17:49
Sperstad S, Lutnaes BF, Stormo SK, Liaaen-Jensen S, Landfald B (2006) Torularhodin and torulene are the major contributors to the carotenoid pool of marine Rhodosporidium babjevae (Golubev). J Ind Microbiol Biotechnol 33:269–273
Keceli TM, Erginkaya Z, Turkkan E, Kaya U (2013) Antioxidant and antibacterial effects of carotenoids extracted from Rhodotorula glutinis strains. Asian J Chem 25(1):42–46
Ungureanu C, Ferdes M (2012) Evaluation of antioxidant and antimicrobial activities of torularhodin. Adv Sci Lett 18(1):50–53
Zhu N, Zhong C, Liu T, Zhu Y, Gou S, Bao H, Yao J, Ni J (2021) Newly designed antimicrobial peptides with potent bioactivity and enhanced cell selectivity prevent and reverse rifampin resistance in Gram-negative bacteria. Eur J Pharm Sci 158:105665
Ungureanu C, Dumitriu C, Popescu S, Enculescu M, Tofan V, Popescu M, Pirvua C (2016) Enhancing antimicrobial activity of TiO2/Ti by torularhodin bioinspired surface modifcation. Bioelectrochemistry 107:14–24
Fourati M, Smaoui S, Ben Hlima H, Elhadef K, Chakchouk MA, Mellouli L (2020) Variability in phytochemical contents and biological potential of pomegranate (Punica granatum) peel extracts: toward a new opportunity for minced beef meat preservation. J Food Qual 2020:1–14
Sakaki H, Nochide H, Komemushi S, Miki W (2002) Effect of active oxygen species on the productivity of torularhodin byRhodotorulaglutinisNo. 21. J Biosci Bioeng 93(3):338–340
Zoz L, Carvalho JC, Soccol VT, Casagrande TC, Cardoso L (2014) Torularhodin and torulene: bioproduction, properties and prospective applications in food and cosmetics-a review. Braz Arch Biol Technol 58:278–288
Joshi-Navare K, Prabhune AA (2013) biosurfactant-sophorolipid acts in synergy with antibiotics to enhance their efficiency. Biomed Res Int 2013:1–8
Gudiña EJ, Teixeira JA, Rodrigues LR (2016) Biosurfactants produced by marine microorganisms with therapeutic applications. Mar Drugs 14:38
Aldossary SA (2019) Review on pharmacology of Cisplatin: clinical use, toxicity and mechanism of resistance of Cisplatin. Biochem Pharmacol J 12(1):7–15
Zhang Q, Lu QB (2021) New combination chemotherapy of cisplatin with an electron-donating compound for treatment of multiple cancers. Sci Rep 11(1):788
Acknowledgements
We would like to express our gratitude to the whole team of the CBS-unit of analysis for their help in the analytic part. We are also grateful to Pr. Mohamed Chemkha and Mr. Nidhal Baccar (LBPE, CBS) for their help in the FTIR analysis.
Funding
This work received financial support from the Ministry of Higher Education and Scientific Research, Tunisia, granted to the Laboratory of Molecular Biotechnology of Eukaryotes, through the Young Researcher Encouragement Project (19PEJC05-10).
Author information
Authors and Affiliations
Contributions
Resources, Conceptualization, Methodology, Software, Data curation, Writing- Original draft preparation and Achievement of manipulations [Mohamed Guerfali]. Methodology, Formal analysis [Ines Ayadi]. Achievement of manipulations [Wajdi Ayadi], cells culture and cytotoxicity test assay. Achievement of manipulations [Slim Smaoui and Elhadef Khaoula], antimicrobial and antioxidant activities monitoring. Formal analysis [Hatem Zaghden]. Software [Lobna Jlaiel], analytical part. Achievement of manipulations [Emna Sahli], flow cytometry. Visualization, Investigation [Hafedh Belghith]. Validation; Writing- Reviewing and Editing, Project administration, Supervision [Ali Gargouri].
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Biodiesel-derived crude glycerol for metabolites production by Y-SL7 strain.
• Fed-batch fermentation to enhance lipids and carotenoids yields.
• Membrane permeability and metabolites recovery.
• Structural characterization of neutral lipids, PEFA, and carotenoids.
• Potential biotechnological applications of Y-SL7 metabolites.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guerfali, M., Ayadi, I., Ayadi, W. et al. Concomitant production of multifunctional metabolites on biodiesel-derived crude glycerol by the oleaginous yeast Rhodotorula babjevae Y-SL7. Biomass Conv. Bioref. 14, 10237–10250 (2024). https://doi.org/10.1007/s13399-022-03028-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03028-5