Abstract
Renewable, scalable and efficient porous adsorbent for removal of Alizarin Red dye from aqueous solution has been developed. Chicken feathers as undesirable bio-waste was recycled via one pot carbonization step to porous carbon. Then, its surface was smartly functionalized with thin layer of polypyrrole via environmentally friendly vapor phase polymerization method. This creates a new and smart porous adsorbent for efficient removal of anionic Alizarin Red dye from aqueous solution. Hence, the obtained adsorbent has positive surface centers and superior porous structure compared to unmodified porous carbon. The developed adsorbent achieved specific surface area and total pore volume of 856 m2 g−1 and 0.56 cm3 g−1 compared to 208 m2 g−1 and 0.23 cm3 g−1 for unmodified carbon, respectively. The smart porous adsorbent achieved excellent removal efficiency of Alizarin Red dye from aqueous solution of 97% compared to 41% for unmodified carbon and achieving maximum uptake capacity of 157 mg g−1. The impact of adsorption factors of pH, adsorbent dose, initial concentration of dye, temperature and contact time was studied. The adsorption kinetic parameters of the adsorption of dye on adsorbent surface were studied and isotherm of the reaction was fitted well to the Langmuir model. Additionally, the thermodynamic parameters of free energy, enthalpy and entropy of adsorption were studied and confirm the adsorption reaction is of endothermic behavior. Moreover, the adsorption mechanism of dye on developed adsorbent surface and reusability of adsorbent were studied.
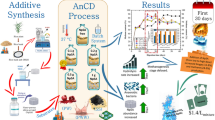
Graphical abstract











Similar content being viewed by others
Data availability
Available based on request.
References
Sreeprasad T, Maliyekkal SM, Pradeep T (2011) Reduced graphene oxide-metal/metal oxide composites: facile synthesis and application in water purification. J Hazard Mater 186:921–931. https://doi.org/10.1016/j.jhazmat.2010.11.100
Patrick W. Egypt looks to the sea to meet its need for water, The National. URL https://www.thenational.ae/business/egypt-looks-to-the-sea-to-meet-its-need-for-water- 1.58966 2017 (accessed 15.02.2022)
Sun WH, Ang HM, Tadé MO (2013) Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem Eng J 226:336–347. https://doi.org/10.1016/j.cej.2013.04.070
Eltaweil AS, Omer AM, El-Aqapa HG, Gaber NM, Attia NF, El-Subruiti GM, Mohy-Eldin MS, Abd El-Monaem EM (2021) Chitosan based adsorbents for the removal of phosphate and nitrate: a critical review. Carbohyd Polym 274:118671. https://doi.org/10.1016/j.carbpol.2021.118671
Galal A, Zaki MM, Atta NF, Samaha SH, Nasr HE, Attia NF (2021) Electroremoval of copper ions from aqueous solutions using chemically synthesized polypyrrole on polyester fabrics. J Water Proc Eng 43:102287. https://doi.org/10.1016/j.jwpe.2021.102287
Gao C, Yu X-Y, Xu R-X, Liu J-H, Huang X-J (2012) AlOOH-reduced graphene oxide nanocomposites: one-pot hydrothermal synthesis and their enhanced electrochemical activity for heavy metal ions. ACS Appl Mater Inter 4:4672–4682. https://doi.org/10.1021/am3010434
Zhang L, Yan L, Xu W, Guo X, Cui L, Gao L, Wei Q, Du B (2014) Adsorption of Pb (II) and Hg (II) from aqueous solution using magnetic CoFe2O4-reduced graphene oxide. J Mol Liq 191:177–182. https://doi.org/10.1016/j.molliq.2013.12.015
Saxena R, Saxena M, Lochab A (2020) Recent progress in nanomaterials for adsorptive removal of organic contaminants from wastewater. Chem Select 9:335–353. https://doi.org/10.1002/slct.201903542
Omer AM, Abd El-Monaem EM, El-Subruiti GM, Abd El-Latif MM, Eltaweil AS (2021) Fabrication of easy separable and reusable MIL-125(Ti)/MIL-53(Fe) binary MOF/CNT/Alginate composite microbeads for tetracycline removal from water bodies. Sci Rep 11:23818. https://doi.org/10.1038/s41598-021-03428-z
Attia NF, Elashery SEA, Zakria AM, Eltaweil AS, Oh H (2021) Recent advances in graphene sheets as new generation of flame-retardant materials. Mater Sci Eng: B. 274:115460. https://doi.org/10.1016/j.mseb.2021.115460
Prajapati AK, Verma P, Singh S, Mondal MK (2022) Adsorption-desorption surface bindings, kinetics, and mass transfer behavior of thermally and chemically treated great millet husk towards Cr (VI) removal from synthetic wastewater. Ad Sci Technol 2022:3956977. https://doi.org/10.1155/2022/3956977
Kannaujiya MC, Gupta GK, Mandal T, Mondal MK (2022) (2022) Adsorption of acid yellow 2GL dye from simulated water using brinjal waste. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-02131-3
Prajapati AK, Mondal MK (2021) Novel green strategy for CuO–ZnO–C nanocomposites fabrication using marigold (Tagetes spp.) flower petals extract with and without CTAB treatment for adsorption of Cr (VI) and Congo red dye. J Environ Manage. 290:112615. https://doi.org/10.1016/j.jenvman.2021.112615
Prajapati AK, Mondal MK (2020) Comprehensive kinetic and mass transfer modeling for methylene blue dye adsorption onto CuO nanoparticles loaded on nanoporous activated carbon prepared from waste coconut shell. J Mol Liq 307:112949. https://doi.org/10.1016/j.molliq.2020.112949
Prajapati AK, Mondal MK (2021) Development of CTAB modified ternary phase α-Fe2O3-Mn2O3-Mn3O4 nanocomposite as innovative super-adsorbent for Congo red dye adsorption. J Environ Chem Eng 9:104827. https://doi.org/10.1016/j.jece.2020.104827
Amin NK (2009) Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: adsorption equilibrium and kinetics. J Hazard Mater 165:52–62. https://doi.org/10.1016/j.jhazmat.2008.09.067
Mittal J (2020) Permissible synthetic food dyes in India. Resonance 25:567–577. https://doi.org/10.1007/s12045-020-0970-6
Wong S, Ngadi N, Inuwa IM, Hassan O (2018) Recent advances in applications of activated carbon from biowaste for wastewater treatment: a short review. J Cleaner Prod 175:361–375. https://doi.org/10.1016/j.jclepro.2017.12.059
Nambela L, Haule LV, Mgani Q (2020) A review on source, chemistry, green synthesis and application of textile colorants. J Cleaner Prod 246:119036. https://doi.org/10.1016/j.jclepro.2019.119036
Fu F, Gao Z, Gao L, Li D-S (2011) Effective adsorption of anionic dye, Alizarin Red S from aqueous solutions on activated clay modified by iron oxide. Ind Eng Chem Res 50:9712–9717. https://doi.org/10.1021/ie200524b
Al-Dǎamy MA, Al-Shemary RQ (2018) Removal of Alizarin Red dye from aqueous solution with bio Sorption technique using snail shell as low cost Adsorbent. J Glob Pharma Technol 10:422–430
Teotia M, Mittal A, Rakesh Kumar Soni RK (2019) Light-mediated thermoset polymers. Materials for Biomedical Engineering Thermoset and Thermoplastic Polymers 57-103. https://doi.org/10.1016/B978-0-12-816874-5.00003-7.Ch3
Mittal J (2021) Recent progress in the synthesis of layered double hydroxides and their application for the adsorptive removal of dyes: a review. J Environ Manage 295:113017. https://doi.org/10.1016/j.jenvman.2021.113017
Mittal A, Mittal J (2015) Hen feather: a remarkable adsorbent for dye removal. 409–457. https://doi.org/10.1002/9781118721001.ch11
Arora C, Soni S, Bajpai PK, Mittal J, Mariyam A (2021) Dye removal from waste water using metal-organic frameworks. 375–393. https://doi.org/10.1016/B978-0-12-822263-8.00014-2 Ch 14.
Mittal J, Mariyam A, Sakina F, Baker RT, Sharma AK, Mittal A (2021) Batch and bulk adsorptive removal of anionic dye using metal/halide-free ordered mesoporous carbon as adsorbent. J Cleaner Prod 321:129060. https://doi.org/10.1016/j.jclepro.2021.129060
Mittal J, Ahmad R, Ejaz MO, Mariyam A, Mittal A (2021) A novel, eco-friendly bio-nanocomposite (Alg-Cst/Kal) for the adsorptive removal of crystal violet dye from its aqueous solutions. Int J Phytoremed. https://doi.org/10.1080/15226514.2021.1977778
Mariyam A, Mittal J, Sakina F, Baker RT, Sharma AK, Mittal A (2021) Efficient batch and fixed-bed sequestration of a basic dye using a novel variant of ordered mesoporous carbon as adsorbent. Arab J Chem 14:103186. https://doi.org/10.1016/j.arabjc.2021.103186
Mittal J, Ahmad R, Mittal A (2021) Kahwa tea (Camellia sinensis) carbon - a novel and green low-cost adsorbent for the sequestration of titan yellow dye from its aqueous solutions. Desal Water Treat 227:404–411. https://doi.org/10.5004/dwt.2021.27284
Patela A, Sonia S, Mittal J, Mittal A, Arora C (2021) Sequestration of crystal violet from aqueous solution using ash of black turmeric rhizome. Desal Water Treat 220:342–352. https://doi.org/10.5004/dwt.2021.26911
Mittal J, Ahmad R, Mariyam A, Gupta VK, Mittal A (2021) Expeditious and enhanced sequestration of heavy metal ions from aqueous environment by papaya peel carbon: a green and low-cost adsorbent. Desal Water Treat 210:365–376. https://doi.org/10.5004/dwt.2021.26562
Hu N, Zhang K, Zhao Y, Zhang Z, Li H (2020) Flotation-based dye removal system: sweet potato protein fabricated from agro-industrial waste as a collector and frother. J Cleaner Prod 269:122121. https://doi.org/10.1016/j.jclepro.2020.122121
Attia NF, Park J, Oh H (2018) Facile tool for green synthesis of graphene sheets and their smart free-standing UV protective film. Appl Surf Sci 458:425–430. https://doi.org/10.1016/j.apsusc.2018.07.066
Jung M, Park J, Lee K, Attia NF, Oh H (2020) Effective synthesis route of renewable nanoporous carbon adsorbent for high energy gas storage and CO2/N2 selectivity. Renew Energy 161:30–42. https://doi.org/10.1016/j.renene.2020.06.125
Erdogan Y, Isik B, Ugraskan V, Fatih Cakar (2022) Effective and fast removal of crystal violet dye from aqueous solutions using Rumex acetosella: isotherm, kinetic, thermodynamic studies, and statistical analysis. Biomass Conv. Bioref. https://doi.org/10.1007/s13399-022-02349-9
Ibrahim M, Souleiman M, Salloum A (2021) Methylene blue dye adsorption onto activated carbon developed from Calicotome villosa via H3PO4 activation. Biomass Conv. Bioref. https://doi.org/10.1007/s13399-021-02027-2
Ugraskan V, Isik B, Yazici O, Cakar F (2022) Removal of Safranine T by a highly efficient adsorbent (Cotinus Coggygria leaves): isotherms, kinetics, thermodynamics, and surface properties. Surf Interf 28:101615. https://doi.org/10.1016/j.surfin.2021.101615
Singh S, Prajapati AK, Chakraborty JP, Monoj Kumar Mondal MK (2021) Adsorption potential of biochar obtained from pyrolysis of raw and torrefied Acacia nilotica towards removal of methylene blue dye from synthetic wastewater. Biomass Conv. Bioref. https://doi.org/10.1007/s13399-021-01645-0
Xia L, Zhou S, Zhang C, Fu Z, Wang A, Zhang Q, Wang Y, Liu X, Wang X, Xu W (2020) Environment-friendly Juncus effusus-based adsorbent with a three-dimensional network structure for highly efficient removal of dyes from wastewater. J Cleaner Prod 259:120812. https://doi.org/10.1016/j.jclepro.2020.120812
Sevilla M, Mokaya R (2014) Energy storage applications of activated carbons: supercapacitors and hydrogen storage. Energy Environ Sci 7:1250–1280. https://doi.org/10.1039/C3EE43525C
Attia NF, Lee SM, Kim HJ, Geckeler KE (2013) Nanoporous carbon- templated silica nanoparticles: preparation, effect of different carbon precursors, and their hydrogen storage adsorption. Micro Meso Mater 173:139–146. https://doi.org/10.1016/j.micromeso.2013.02.016
Park J, Attia NF, Jung M, Lee M, Lee K, Chung J, Oh H (2018) Sustainable nanoporous carbon for CO2, CH4, N2, H2 adsorption and CO2/CH4 and CO2/N2 separation. Energy 158:9–16. https://doi.org/10.1016/j.energy.2018.06.010
Arami-Niya N, Rufford TE, Zhu Z (2016) Nitrogen-doped carbon foams synthesized from banana peel and zinc complex template for adsorption of CO2, CH4 and N2. Energy Fuels 30:7298–7309. https://doi.org/10.1021/acs.energyfuels.6b00971
Park J, Cho SY, Jung M, Lee K, Nah YC, Attia NF, Oh H (2021) Efficient synthetic approach for nanoporous adsorbents capable of pre and post combustion CO2 capture and selective gas separation. J CO2 Utili 45:101404. https://doi.org/10.1016/j.jcou.2020.101404
Frag EY, El-Zaher NA, Elashery SEA (2020) Carbon thick sheet potentiometric sensor for selective determination of silver ions in X-ray photographic film. Microchem J 155:104750. https://doi.org/10.1016/j.microc.2020.104750
Frag EY, Mohamed NM, Elashery SEA (2021) Exploitation of o-benzoyl benzoic acid as an efficient electroactive material for selective determination of Cr (III) ions in pharmaceutical samples and industrial waste water using carbon sensor. Anal Chim Acta 1154:338322. https://doi.org/10.1016/j.aca.2021.338322
Ghosh R, Stewart R (2014) Keratinous materials as novel absorbent systems for toxic pollutants. Defence Sci J 64:209–221. https://doi.org/10.14429/dsj.64.7319
Belarmino DD, Ladchumananandasivam R, Belarmino LD, Pimentel JRD, Rocha BGD, Galvao AO, Andrade SMB (2012) Physical and morphological structure of chicken feathers (Keratin Biofiber) in natural, chemically and thermally modified forms. Mater Sci Appl 3:887–893. https://doi.org/10.4236/msa.2012.312129
Tesfaye T, Sithole B, Ramjugernath D, Chunlall V (2017) Valorisation of chicken feathers: characterisation of physical properties and morphological structure. J Cleaner Prod 149:349–365. https://doi.org/10.1016/j.jclepro.2017.02.112
Gao L, Li R, Sui X, Li R, Chen C, Chen Q (2014) Conversion of chicken feather waste to N-doped carbon nanotubes for the catalytic reduction of 4-nitrophenol. Environ Sci Technol 48:10191–10197. https://doi.org/10.1021/es5021839
Tuna A, Okumuş Y, Çelebi H, Seyhan AT (2015) Thermochemical conversion of poultry chicken feather fibers of different colors into microporous fibres. J Anal Appl Pyrolysis 115:112–124. https://doi.org/10.1016/j.jaap.2015.07.008
Mittal A, Thakur V, Gajbe V (2013) Adsorptive removal of toxic azo dye Amido Black 10B by hen feather. Environ Sci Pollution Res 20:260–269. https://doi.org/10.1007/s11356-012-0843-y
Mittal J, Thakur V, Mittal A (2013) Batch removal of hazardous azo dye Bismark Brown R using waste material hen feather. Ecol Eng 60:249–253. https://doi.org/10.1016/j.ecoleng.2013.07.025
Mittal A, Thakur V, Gajbe V (2012) Evaluation of adsorption characteristics of an anionic azo dye Brilliant Yellow onto hen feathers in aqueous solutions. Environ Sci Pollution Res 19:2438–2447. https://doi.org/10.1007/s11356-012-0756-9
Mittal A, Kurup L, Mittal J (2007) Freundlich and Langmuir adsorption isotherms and kinetics for the removal of Tartrazine from aqueous solutions using hen feathers. J Hazard Mater 146:243–248. https://doi.org/10.1016/j.jhazmat.2006.12.012
Gupta VK, Mittal A, Kurup L, Mittal J (2006) Adsorption of a hazardous dye, erythrosine, over hen feathers. J Colloid Interf Sci 304:52–57. https://doi.org/10.1016/j.jcis.2006.08.032
Mittal A (2006) Adsorption kinetics of removal of a toxic dye, Malachite Green, from wastewater by using hen feathers. J Hazard Mater 133:196–202. https://doi.org/10.1016/j.jhazmat.2005.10.017
Mittal A (2006) Use of hen feathers as potential adsorbent for the removal of a hazardous dye, Brilliant Blue FCF, from wastewater. J Hazard Mater 128:233–239. https://doi.org/10.1016/j.jhazmat.2005.08.043
Alok Mittal A, Thakur V, Mittal J, Vardhan H (2014) Process development for the removal of hazardous anionic azo dye Congo red from wastewater by using hen feather as potential adsorbent Des. Water Treat 52:227–237. https://doi.org/10.1080/19443994.2013.785030
MacDiarmid AG, Epstein AJ (1995) Secondary doping in polyaniline. Synth Met 69:85–92. https://doi.org/10.1016/0379-6779(94)02374-8
Geniès EM, Boyle A, Lapkowski M, Tsintavis C (1990) Polyaniline: a historical survey. Synth Met 36:139–182. https://doi.org/10.1016/0379-6779(90)90050-U
Attia NF, Geckeler KE (2013) Polyaniline–polypyrrole composites with enhanced hydrogen storage capacities. Macromol Rapid Commun 34:931–937. https://doi.org/10.1002/marc.201300060
Attia NF, Geckeler KE (2013) Polyaniline as a material for hydrogen storage applications. Macromol Rapid Commun 34:1043–1055. https://doi.org/10.1002/marc.201300255
Attia NF, Abdel Hady K, Elashery SEA, Hashem HM, Oh H, Refaat AM, Abdel Hady A (2020) Greener synthesis route and characterization of smart hybrid graphene based thin films. Surf Interf 21:100681. https://doi.org/10.1016/j.surfin.2020.100681
Jung M, Park J, Cho SY, Elashery SEA, Attia NF, Oh H (2021) Flexible carbon sieve based on nanoporous carbon cloth for efficient CO2/CH4 separation. Surf Interf 23:100960. https://doi.org/10.1016/j.surfin.2021.100960
Lü Z, Hu F, Li H, Zhang X, Yu S, Liu M, Gao C (2019) Composite nanofiltration membrane with asymmetric selective separation layer for enhanced separation efficiency to anionic dye aqueous solution. J Hazard Mater 368:436–443. https://doi.org/10.1016/j.jhazmat.2019.01.086
Adeogun AI, Balakrishnan RB (2016) Electrocoagulation removal of anthraquinone dye Alizarin Red S from aqueous solution using aluminum electrodes: kinetics, isothermal and thermodynamics studies. J Electrochem Sci Eng 6:199–213. https://doi.org/10.5599/jese.290
Ma SS, Gang Zhang Y (2016) Electrolytic removal of Alizarin Red S by Fe/Al composite hydrogel electrode for electrocoagulation toward a new wastewater treatment. Environ Sci Pollut Res 23:22771–22782. https://doi.org/10.1007/s11356-016-7483-6
Mukherjee T, Das P, Ghosh SK, Rahaman M (2019) Removal of Alizarin Red S from wastewater: optimizing the process parameters for electrocoagulation using Taguchi method. Waste Water Rec. Manage. 239–249. Singapore. https://doi.org/10.1007/978-981-13-2619-6_19
Badran I, Khalaf R (2020) Adsorptive removal of Alizarin dye from wastewater using maghemite nanoadsorbents. Sep Sci Technol 45:1–16. https://doi.org/10.1080/01496395.2019.1634731
Park J, Jung M, Jang H, Lee K, Attia NF, Oh H (2018) A facile synthesis tool of nanoporous carbon for promising H2, CO2, and CH4 sorption capacity and selective gas separation. J Mater Chem A 6:23087–23100. https://doi.org/10.1039/C8TA08603F
Elashery SEA, Attia NF, Omar MM, Tayea HMI (2019) Cost-effective and green synthesized electroactive nanocomposite for high selective potentiometric determination of clomipramine hydrochloride. Microchem J 151:104222. https://doi.org/10.1016/j.microc.2019.104222
Attia NF, Jung M, Park J, Cho SY, Oh H (2020) Facile synthesis of hybrid porous composites and its porous carbon for enhanced H2 and CH4 storage. Int J Hydrogen Energy 45:32797–32807. https://doi.org/10.1016/j.ijhydene.2020.03.004
Attia NF, Ahmed HE, Yehia D, Hassan MA, Ziddan Y (2017) Novel synthesis of nanoparticles based back coating flame retardant materials for historic textile fabrics conservation. J Indust Text 46:1379–1392. https://doi.org/10.1177/1528083715619957
Attia NF, Eid AM, Soliman MA, Nagy M (2018) Exfoliation and decoration of graphene sheets with silver nanoparticles and their antibacterial properties. J Polym Environ 26:1072–1077. https://doi.org/10.1007/s10924-017-1014-5
Attia NF, Mousa M (2017) Synthesis of smart coating for furniture textile and their flammability and hydrophobic properties. Prog Org Coat 110:204–209. https://doi.org/10.1016/j.porgcoat.2017.04.035
Barrett EP, Joyner LS, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380. https://doi.org/10.1021/ja01145a126
Sevilla M, Valle-Vigon P, Fuertes AB (2011) N-doped polypyrrole-based porous carbons for CO2 capture. Adv Funct Mater 21:2781–2787. https://doi.org/10.1002/adfm.201100291
Zhang X, Zhang J, Song W, Liu Z (2006) Controllable synthesis of conducting polypyrrole nanostructures. J Phys Chem B 110:1158–1165. https://doi.org/10.1021/jp054335k
Nabid MR, Entezami AA (2004) A novel method for synthesis of water-soluble polypyrrole with horseradish peroxidase enzyme. J Appl Polym Sci 94:254–258. https://doi.org/10.1002/app.20882
Peng WC, Chen Y, Li XY (2016) MoS2/reduced graphene oxide hybrid with CdS nanoparticles as a visible light-driven photocatalyst for the reduction of 4-nitrophenol. J Hazard Mater 309:173–179. https://doi.org/10.1016/j.jhazmat.2016.02.021
Attia NF, Lee SM, Kim HJ, Geckeler KE (2015) Preparation of polypyrrole nanoparticles and their composites: effect of electronic properties on the hydrogen adsorption. Polym Int 64:696–703. https://doi.org/10.1002/er.3095
Cheng Q, Pablinek V, Li CZ, Lengalova A, He Y, Saha P (2006) Synthesis and structural properties of polypyrrole/nano-Y2O3 conducting composite. Appl Surf Sci 253:1736–1740. https://doi.org/10.1016/j.apsusc.2006.03.004
Yang X, Li L (2010) Polypyrrole nanofibers synthesized via reactive template approach and their NH3 gas sensitivity. Synth Met 160:1365–1367. https://doi.org/10.1016/j.synthmet.2010.04.015
Stejskal J, Trchova M, Ananieva IA, Janca J, Prokes J, Fedorova S, Sapurina I (2004) Poly(aniline-co-pyrrole): powders, films, and colloids; Thermophoretic mobility of colloidal particles. Synth Met 146:29–36. https://doi.org/10.1016/j.synthmet.2004.06.013
Romero AJF, Cascales JJL, Otero F (2005) In situ FTIR spectroscopy study of the break-in phenomenon observed for PPy/PVS films in acetonitrile. J Phys Chem B 109:21078–21085. https://doi.org/10.1021/jp054026u
Tu J, Li N, Yuan Q, Wang R, Geng WC, Li Y, Zhang T, Li X (2009) Humidity-sensitive property of Fe2? doped polypyrrole. Synth Met 159:2469–2473. https://doi.org/10.1016/j.synthmet.2009.08.014
Attia NF (2017) Organic nanoparticles as promising flame-retardant materials for thermoplastic polymers. J Therm Anal Calorim 127:2273–2282. https://doi.org/10.1007/s10973-016-5740-z
Zhang R, Wang X, Qi GC, Li BH, Song ZH, Jiang HB, Zhang XH, Qiao JL (2016) A novel N-doped porous carbon microsphere composed of hollow carbon nanospheres. Carbon 96:864–870. https://doi.org/10.1016/j.carbon.2015.10.045
Cheng P, Li T, Yu H, Zhi L, Liu ZH, Lei ZB (2016) Biomass-derived carbon fiber aerogel as a binder-free electrode for high-rate supercapacitors. J Phys Chem C 120:2079–2086. https://doi.org/10.1021/acs.jpcc.5b11280
Sing KSW, Everett DH, Haul RA, Moscou WL, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57:603–612. https://doi.org/10.1351/pac198557040603
Fayazi M, Ghanei-Motlagh M, Taher MA (2015) The adsorption of basic dye (Alizarin Red S) from aqueous solution onto activated carbon/γ-Fe2O3 nano-composite: kinetic and equilibrium studies. Mater Sci Semicond Process 40:35–43. https://doi.org/10.1016/j.mssp.2015.06.044
Gholivand MB, Yamini Y, Dayeni M, Seidi S, Tahmasebi E (2015) Adsorptive removal of Alizarin Red-S and Alizarin Yellow GG from aqueous solutions using polypyrrole-coated magnetic nanoparticles. J Environ Chem Eng 3:529–540. https://doi.org/10.1016/j.jece.2015.01.011
Machado FM, Carmalin SA, Lima EC, Dias SL, Prola LD, Saucier C, Jauris M, Zanella I, Fagan SB (2016) Adsorption of Alizarin Red S dye by carbon nanotubes: an experimental and theoretical investigation. J Phys Chem C 120:18296–18306. https://doi.org/10.1021/acs.jpcc.6b03884
Narayanan AL, Narayanamurthy P, Solomon JS (2015) Thermodynamics and kinetics of adsorption of alizarin yellow from aqueous solutions on saccharum spontaneum. Int J Eng Appl Sci 2:63–69
Diab MA, Attia NF, Attia AS, El-Shahat MF (2020) Green synthesis of cost-effective and efficient nanoadsorbents based on zero and two dimensional nanomaterials for Zn2+ and Cr3+ removal from aqueous solutions. Synth Met 265:116411. https://doi.org/10.1016/j.synthmet.2020.116411
Ahmed AA, Hameed BH, Aziz N (2007) Adsorption of direct dye on palm ash: kinetic and equilibrium modeling. J Hazard Mater 141:70–76. https://doi.org/10.1016/j.jhazmat.2006.06.094
Mckay G, Ho YS (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Li H, Hu J, Wang C, Wang X (2017) Removal of amoxicillin in aqueous solution by a novel chicken feather carbon: kinetic and equilibrium studies. Water Air Soil Pollut 228:201. https://doi.org/10.1007/s11270-017-3385-6
Malik PK (2004) Dye removal from wastewater using activated carbon developed from sawdust: adsorption and kinetics. J Hazard Mater 113:81–88. https://doi.org/10.1016/j.jhazmat.2004.05.022
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Santi Eng Div ASCE 89:31–59. https://doi.org/10.1061/JSEDAI.0000430
Salem MA (2010) The role of polyaniline salts in the removal of direct blue 78 from aqueous solution: a kinetic study. React Funct Polym 70:707–714. https://doi.org/10.1016/j.reactfunctpolym.2010.07.001
Kannan K, Sundaram MM (2001) Kinetics and mechanism of removal of methylene blue by adsorption on various carbons-a comparative study. Dyes Pig 51:25–40. https://doi.org/10.1016/S0143-7208(01)00056-0
Murcia-Salvador A, Pellicer JA, Fortea MI, Gómez-López VM, Rodríguez-López MI, Núñez-Delicado E, Gabaldón JA. Adsorption of direct blue 78 using chitosan and cyclodextrins as adsorbents. Polymers. 11: 1003. https://doi.org/10.3390/polym11061003
Attia NF, Diab MA, Attia AS, El-Shahat MF (2021) Greener approach for fabrication of antibacterial graphene-polypyrrole nanoparticle adsorbent for removal of Mn2+ from aqueous solution. Synth Met 282:116951. https://doi.org/10.1016/j.synthmet.2021.116951
Khayyun TS, Mseer AH (2019) Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl Water Sci 9:170. https://doi.org/10.1007/s13201-019-1061-2
Bhatt AS, Sakaria PL, Vasudevan M, Pawar RR, Sudheesh N, Bajaj HC, Mody HM (2012) Adsorption of an anionic dye from aqueous medium by organoclays: equilibrium modeling, kinetic and thermodynamic exploration. RSC Adv 2:8663–8671. https://doi.org/10.1039/C2RA20347B
Author information
Authors and Affiliations
Contributions
Nour F. Attia: conceptualization, methodology, writing—original draft preparation, reviewing and editing supervision. Sabry M. Shaltout: methodology, investigation, data curation and writing. Ibrahim A. Salem: software and data curation. Ahmed B. Zaki: software and data curation. M. H. El-Sadek: visualization and investigation. Mohamed A. Salem: visualization, investigation, data curation, project administration, reviewing and editing supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Attia, N.F., Shaltout, S.M., Salem, I.A. et al. Sustainable and smart hybrid nanoporous adsorbent derived biomass as efficient adsorbent for cleaning of wastewater from Alizarin Red dye. Biomass Conv. Bioref. 14, 4989–5004 (2024). https://doi.org/10.1007/s13399-022-02763-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02763-z