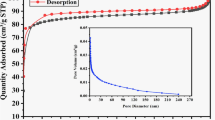
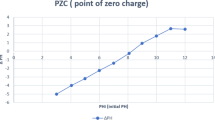
Abstract
The present study focuses on using jackfruit seed waste (JSW) for bioremediation of heavy toxic metals from water. The fruit is abundantly grown in India and Far East countries and produces myriad tons of seeds. A simple procedure is developed in the laboratory by thermally activating JSW by orthophosphoric acid at 500 °C. Surface morphology, porosity, and structural analyses of the resultant biochar were conducted. The activated biochar was applied for batch adsorption of several heavy metal ions: Fe(III), Cd(II), Cu(II), Pb(II), and Mn(VII) at pH 7. The average heavy metal uptake by activated JSW biochar is 76.4 mg g−1, 79.4 mg g−1, 97.9 mg g−1, 79.9 mg g−1, and 79.8 mg g−1 for Fe(III), Pb(II), Cu(II), Cd(II), and Mn(VII), respectively. The experimental conditions were optimized to remove heavy metals at neutral pH. The adsorption process was exothermic, and Langmuir and pseudo-second-order kinetic study models were best fitted. The observation was in close agreement with the experimental data. Thermal and structural characterization of biochar at post-adsorption analyses was conducted. The study has distinguished features of simplicity, cost-effectiveness, and viability compared to many of the literature-reported biomass waste.
Graphical abstract











Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, Nazia Hossain, upon reasonable request.
References
Abbas A, Al-Amer AM, Laoui T, Al-Marri MJ, Nasser MS, Khraisheh M, Atieh MA (2016) Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Sep Purif Technol 157:141–161
Abid MK, Ibrahim HB, Zulkifli SZ (2019) Synthesis and characterization of biochar from peel and seed of jackfruit plant waste for the adsorption of copper metal ion from water. Res J Pharm Technol 12(9):4182–4188
Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang M-Q (2021) Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics 9(3):42
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112(10):5073–5091
Arán D, Antelo J, Fiol S, Macías F (2016) Influence of feedstock on the copper removal capacity of waste-derived biochars. Bioresour Technol 212:199–206
Arshadi M, Amiri MJ, Mousavi S (2014) Kinetic, equilibrium and thermodynamic investigations of Ni (II), Cd (II), Cu (II) and Co (II) adsorption on barley straw ash. Water Resour Ind 6:1–17
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12
Cha JS, Park SH, Jung S-C, Ryu C, Jeon J-K, Shin M-C, Park Y-K (2016) Production and utilization of biochar: a review. J Ind Eng Chem 40:1–15
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88(11):1707–1719
Cui L, Chen T, Yin C, Yan J, Ippolito JA, Hussain Q (2019) Mechanism of adsorption of cadmium and lead ions by iron-activated biochar. BioResources 14(1):842–857
De Souza JVTM, Diniz KM, Massocatto CL, Tarley CRT, Caetano J, Dragunski DC (2012) Removal of Pb (II) from aqueous solution with orange sub-products chemically modified as biosorbent. BioResources 7(2):2300–2318
Fahmi AH, Samsuri AW, Jol H, Singh D (2018) Bioavailability and leaching of Cd and Pb from contaminated soil amended with different sizes of biochar. R Soc Open Sci 5(11):181328
Farghali A, Bahgat M, Allah AE, Khedr M (2013) Adsorption of Pb (II) ions from aqueous solutions using copper oxide nanostructures. Beni-Suef Univ J Basic Appl Sci 2(2):61–71
Haileslassie T, Gebremedhin K (2015) Hazards of heavy metal contamination in ground water. Int J Technol Enhanc Emerg Eng Res 3(2):1–6
Hameed B (2009) Removal of cationic dye from aqueous solution using jackfruit peel as non-conventional low-cost adsorbent. J Hazard Mater 162(1):344–350
Hossain N, Nizamuddin S, Griffin G, Selvakannan P, Mubarak NM, Mahlia TMI (2020) Synthesis and characterization of rice husk biochar via hydrothermal carbonization for wastewater treatment and biofuel production. Sci Rep 10(1):1–15
Hossain N, Nizamuddin S, Selvakannan P, Griffin G, Madapusi S, Shah K (2022) The effect of KOH activation and Ag nanoparticle incorporation on rice husk-based porous materials for wastewater treatment. Chemosphere 291:132760
Hossain N, Nizamuddin S, Shah K (2022) Thermal-chemical modified rice husk-based porous adsorbents for Cu (II), Pb (II), Zn (II), Mn (II) and Fe (III) adsorption. J Water Process Eng 46:102620
Idris NSU, Low KH, Koki IB, Kamaruddin AF, Salleh KM, Zain SM (2017) Hemibagrus sp. as a potential bioindicator of hazardous metal pollution in Selangor River. Environ Monit Assess 189(5):220
Kołodyńska D, Krukowska J, Thomas P (2017) Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem Eng J 307:353–363
Kosolsaksakul P, Oliver IW, Graham MC (2018) Evaluating cadmium bioavailability in contaminated rice paddy soils and assessing potential for contaminant immobilisation with biochar. J Environ Manage 215:49–56
Lee S, Han J, Ro H-M (2022) Mechanistic insights into Cd (II) and As (V) sorption on Miscanthus biochar at different pH values and pyrolysis temperatures. Chemosphere 287:132179
Lokman IM, Rashid U, Taufiq-Yap YH (2016) Meso-and macroporous sulfonated starch solid acid catalyst for esterification of palm fatty acid distillate. Arab J Chem 9(2):179–189
Madruga MS, de Albuquerque FSM, Silva IRA, do Amaral DS, Magnani M, Neto VQ (2014) Chemical, morphological and functional properties of Brazilian jackfruit (Artocarpus heterophyllus L.) seeds starch. Food Chem 143:440–445
Mehrabi N, Soleimani M, Yeganeh MM, Sharififard H (2015) Parameter optimization for nitrate removal from water using activated carbon and composite of activated carbon and Fe2O3 nanoparticles. RSC Adv 5(64):51470–51482
Melo LC, Coscione AR, Abreu CA, Puga AP, Camargo OA (2013) Influence of pyrolysis temperature on cadmium and zinc sorption capacity of sugar cane straw–derived biochar. BioResources 8(4):4992–5004
Oyetade OA, Nyamori VO, Martincigh BS, Jonnalagadda SB (2016) Nitrogen-functionalised carbon nanotubes as a novel adsorbent for the removal of Cu (II) from aqueous solution. RSC Adv 6(4):2731–2745
Patrulea V, Negrulescu A, Mincea MM, Pitulice LD, Spiridon OB, Ostafe V (2013) Optimization of the removal of copper (II) ions from aqueous solution on chitosan and cross-linked chitosan beads. BioResources 8(1):1147–1165
Premachandra JK, Manoj B, Aberathne S, Warnapura L (2017) Removal of lead from synthetic wastewater using chemically modified jackfruit leaves. Paper presented at the 2017 Moratuwa Engineering Research Conference (MERCon).
Radha E, Gomathi T, Sudha P, Latha S, Ghfar AA, Hossain N (2021) Adsorption studies on removal of Pb(II) and Cd(II) ions using chitosan derived copolymeric blend. Biomass Convers Biorefin 1–16
Richard S, Rajadurai JS, Manikandan V (2016) Influence of particle size and particle loading on mechanical and dielectric properties of biochar particulate-reinforced polymer nanocomposites. Int J Polym Anal Charact 21(6):462–477
Rosli NA, Zawawi MH, Bustami RA, Hipni F, Kamruddin MA (2015) Adsorption of lead using jackfruit peel activated carbon. Paper presented at the Applied Mechanics and Materials.
Rudzinski W, Plazinski W (2009) On the applicability of the pseudo-second order equation to represent the kinetics of adsorption at solid/solution interfaces: a theoretical analysis based on the statistical rate theory. Adsorption 15(2):181–192
Saleh ME, Mahmoud AH, El-Refaey AA (2014) Removal of cadmium from aqueous solution by biochars derived from peanut hull and wheat straw. Adv Environ Biol 399–410
Shahapurkar K, Chenrayan V, Soudagar MEM, Badruddin IA, Shahapurkar P, Elfasakhany A, … Mahlia TMI (2021) Leverage of environmental pollutant crump rubber on the dry sliding wear response of epoxy composites. Polymers 13(17):2894
Shaheen SM, Niazi NK, Hassan NE, Bibi I, Wang H, Tsang DC, … Rinklebe J (2019) Wood-based biochar for the removal of potentially toxic elements in water and wastewater: a critical review. Int Mater Rev 64(4):216–247
Silas S, Sailaja D, Rao G (2017) Use of palm kernel cake as low cost biosorbent for the removal of cadmium from aqueous solution. IOSR J Environ Sci Toxicol Food Technol 11:38–50
Tao S, Wu Z, He X, Ye B-C, Li C (2018) Characterization of biochar prepared from cotton stalks as efficient inoculum carriers for Bacillus subtilis SL-13. BioResources 13(1):1773–1786
Taqui SN, Cs M, Goodarzi MS, Elkotb MA, Khatoon BA, Soudagar MEM, … Ali MA (2021) Sustainable adsorption method for the remediation of crystal violet dye using nutraceutical industrial fenugreek seed spent. Appl Sci 11(16):7635
Taqui SN, Mohan C, Khatoon BA, Soudagar, MEM, Khan T, Mujtaba M, … Pruncu CI (2021) Sustainable adsorption method for the remediation of malachite green dye using nutraceutical industrial fenugreek seed spent. Biomass Convers Biorefin 1–12
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KS (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87(9–10):1051–1069
Uchimiya M, Wartelle LH, Klasson KT, Fortier CA, Lima IM (2011) Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J Agric Food Chem 59(6):2501–2510
Ukkund SJ, Puthiyillam P, Alshehri HM, Goodarzi M, Taqui SN, Anqi AE, … Mir RA (2021) Adsorption method for the remediation of brilliant green dye using halloysite nanotube: isotherm, kinetic and modeling studies. Appl Sci 11(17):8088
Wan S, He F, Wu J, Wan W, Gu Y, Gao B (2016) Rapid and highly selective removal of lead from water using graphene oxide-hydrated manganese oxide nanocomposites. J Hazard Mater 314:32–40
Xie Z, Guan W, Ji F, Song Z, Zhao Y (2014) Production of biologically activated carbon from orange peel and landfill leachate subsequent treatment technology. J Chem 2014
Yaashikaa P, Kumar PS, Jeevanantham S, Saravanan R (2022) A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ Pollut 119035
Yang X, Ng W, Wong BSE, Baeg GH, Wang C-H, Ok YS (2019) Characterization and ecotoxicological investigation of biochar produced via slow pyrolysis: effect of feedstock composition and pyrolysis conditions. J Hazard Mater 365:178–185
Yang Y, Meehan B, Shah K, Surapaneni A, Hughes J, Fouché L, Paz-Ferreiro J (2018) Physicochemical properties of biochars produced from biosolids in Victoria, Australia. Int J Environ Res Public Health 15(7):1459
Yaradoddi JS, Banapurmath NR, Ganachari SV, Soudagar MEM, Mubarak N, Hallad S, … Fayaz H (2020) Biodegradable carboxymethyl cellulose based material for sustainable packaging application. Sci Rep 10(1):1–13
Yaradoddi JS, Banapurmath NR, Ganachari SV, Soudagar MEM, Sajjan AM, Kamat S, … Safaei MR (2022) Bio-based material from fruit waste of orange peel for industrial applications. J Mater Res Technol 17:3186–3197
Zaini MAA, Zhi LL, Hui TS, Amano Y, Machida M (2021) Effects of physical activation on pore textures and heavy metals removal of fiber-based activated carbons. Mater Today Proc 39:917–921
Zama EF, Zhu Y-G, Reid BJ, Sun G-X (2017) The role of biochar properties in influencing the sorption and desorption of Pb (II), Cd (II) and As (III) in aqueous solution. J Clean Prod 148:127–136
Zhao S-X, Ta N, Wang X-D (2017) Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 10(9):1293
Funding
This study was funded by Taif University researchers supporting project number TURSP–2020/40, Taif University, Taif, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclaimer
The facts and views in the manuscript are solely ours, and we are totally responsible for authenticity, validity, and originality. We also declare that this manuscript is our original work, and we have not copied from anywhere else. There is no plagiarism in our manuscript.
Consent for publication
We undertake and agree that the manuscript submitted to this journal has not been published elsewhere and has not been simultaneously submitted to other journals.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khadem, M., Husni Ibrahim, A., Mokashi, I. et al. Removal of heavy metals from wastewater using low-cost biochar prepared from jackfruit seed waste. Biomass Conv. Bioref. 13, 14447–14456 (2023). https://doi.org/10.1007/s13399-022-02748-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02748-y