Abstract
The TG-FTIR and Py-GC/MS techniques are applied to investigate the behaviors and kinetics of the Chlorella pyrenoidosa fodder (CPF) pyrolysis under slow and fast heating, respectively. TG-FTIR results reveal that the CPF pyrolysis mainly occurs in temperature range of 150–600 ℃, where 432 ℃ is responsible for the fastest releases of bio-oil and gases. The NH3 and nitrogenous organic compounds derived from protein decomposition generate over the entire pyrolytic temperature range. According to the Py-GC/MS, the bio-oil has maximum contents of C11-C22 (~ 66.5 wt%) and C4-C10 (~ 99.2%) compounds at 600℃ and 800℃, respectively. Higher pyrolytic temperature is appropriate for bio-oil conversion to gasoline energy potential. Temperature increasing can convert the C11-C22 into C4-C10, attributing to cracking the long carbon chain to a shorter length. The Vyazovkin method and a modified kinetic compensation effects (KCE) model are adopted in model-free procedure to investigate the single-step pyrolysis of CPF. Kinetic results showed that the CPF pyrolysis has an average activation energy of 181.7 kJ/mol. But the activation energy varies greatly with conversion rate from 128 kJ/mol to 438 kJ/mol attributing to mechanism changes. The modified KCE model, which enabled activation energy fluctuations, could optimize the single-step pyrolysis of CPF with reaction order of n = 7.8. A parallel two-step reaction (PTSR) model which assumes the pseudo protein and carbohydrate decompose independently in two-step reaction was developed to depict the multi-step pyrolysis of CPF. This PTSR model could fit the experiments well with very low relative deviation. The activation energies of pseudo protein and carbohydrate were 94.22 kJ/mol and 154.85 kJ/mol in the first-step reaction and 109.37 kJ/mol and 142.84 kJ/mol in the first and second step reaction, respectively. The lipid pyrolysis follows a single-step reaction with activation energy of 156.83 kJ/mol.
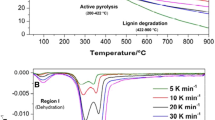
Graphical abstract







Similar content being viewed by others
Data availability
Some or all data, models, or code that support the findings of this study are available from the corresponding author upon reasonable request.
References
I Ali SR Naqvi A Bahadar 2018 Kinetic analysis of Botryococcus braunii pyrolysis using model-free and model fitting methods Fuel 214 369 380
K Anastasakis AB Ross JM Jones 2011 Pyrolysis behaviour of the main carbohydrates of brown macro-algae Fuel 90 598 607
A Anca-Couce C Tsekos S Retschitzegger F Zimbardi A Funke S Banks T Kraia P Marques R Scharler W Jong de N Kienzl 2020 Biomass pyrolysis TGA assessment with an international round robin Fuel 276 118002
R Aniza WH Chen YY Lin KQ Tran JS Chang SS Lam YK Park EE Kwon M Tabatabaei 2021 Independent parallel pyrolysis kinetics of extracted proteins and lipids as well as model carbohydrates in microalgae Appl Energ 300 117372
K Azizi M Keshavarz Moraveji H Abedini Najafabadi 2018 A review on bio-fuel production from microalgal biomass by using pyrolysis method Renew Sustain Energ Rev 82 3046 3059
QV Bach WH Chen 2017a Pyrolysis characteristics and kinetics of microalgae via thermogravimetric analysis (TGA): A state-of-the-art review Bioresour Technol 246 88 100
QV Bach WH Chen 2017b A comprehensive study on pyrolysis kinetics of microalgal biomass Energ Convers Manag 131 109 116
A Bridgwater Aston 2012 Review of fast pyrolysis of biomass and product upgrading Biomass Bioenerg 30 68 94
JA Caballero R Font MM Esperanza 1998 Kinetics of the thermal decomposition of tannery waste J Anal Appl Pyrolysis 47 165 181
J Cai D Xu Z Dong X Yu Y Yang SW Banks AV Bridgwater 2018 Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk Renew Sustain Energ Rev 82 2705 2715
C Chen W Miao C Zhou H Wu 2017 Thermogravimetric pyrolysis kinetics of bamboo waste via Asymmetric Double Sigmoidal (Asym2sig) function deconvolution Bioresour Technol 225 48 57
R Chen Q Li X Xu D Zhang 2019 Pyrolysis kinetics and reaction mechanism of representative non-charring polymer waste with micron particle size Energ Convers Manag 198 111923
W Chen H Yang Y Chen M Xia X Chen H Chen 2017 Transformation of Nitrogen and Evolution of N-Containing Species during Algae Pyrolysis Environ Sci Technol 51 6570 6579
Z Chen Y Li P Li M Wu X Zhang X Zhang G Zhu 2019 Investigations on Cunninghamia Lanceolate Cedar Wood Pyrolysis by Thermogravimetric-Fourier Transform Infrared Analysis and a Modified Discrete Distributed Activation Energy Model Kinetic Method Energ Fuel 33 12499 12507
Q Cheng M Jiang Z Qin S Zhang M Wang J Li 2017 Thermogravimetry Study of the Pyrolytic Characteristics and Kinetics of Fast-Growing Eucalyptus Residue Energ Fuel 31 13675 13681
R Font I Martín-Gullón M Esperanza A Fullana 2001 Kinetic law for solids decomposition. Application to thermal degradation of heterogeneous materials J Anal Appl Pyrolysis 58–59 703 731
C Gai Y Guo N Peng T Liu Z Liu 2016 N-Doped biochar derived from co-hydrothermal carbonization of rice husk and: Chlorella pyrenoidosa for enhancing copper ion adsorption RSC Adv 6 53713 53722
C Gai Y Zhang WT Chen P Zhang Y Dong 2013 Thermogravimetric and kinetic analysis of thermal decomposition characteristics of low-lipid microalgae Bioresour Technol 150 139 148
González E, García MF, Asensio IA, Díaz O, Vera L, González Díaz E (2020) Analysis of the pyrolysis kinetics of wastewater-fed microalgal biomass by a parallel order-based reaction model. Energy Sources, Part A Recover. Util. Environ. Eff., 1–14
Hameed Z, Naqvi S R, Naqvi M, et al. A comprehensive review on thermal coconversion of biomass, sludge, coal, and their blends using thermogravimetric analysis[J]. Journal of Chemistry, 2020
M Hu Z Chen D Guo C Liu B Xiao Z Hu S Liu 2015 Thermogravimetric study on pyrolysis kinetics of Chlorella pyrenoidosa and bloom-forming cyanobacteria Bioresour Technol 177 41 50
F Huang A Tahmasebi K Maliutina J Yu 2017 Formation of nitrogen-containing compounds during microwave pyrolysis of microalgae: Product distribution and reaction pathways Bioresour Technol 245 1067 1074
B Janković B Adnadević J Jovanović 2007 Application of model-fitting and model-free kinetics to the study of non-isothermal dehydration of equilibrium swollen poly (acrylic acid) hydrogel: Thermogravimetric analysis Thermochim Acta 452 106 115
R Kaur P Gera MK Jha T Bhaskar 2018 Pyrolysis kinetics and thermodynamic parameters of castor (Ricinus communis) residue using thermogravimetric analysis Bioresour Technol 250 422 428
SA Khan I Ali SR Naqvi 2021 Investigation of slow pyrolysis mechanism and kinetic modeling of Scenedesmus quadricauda biomass J Anal Appl Pyrol 158 105149
KM Kwon IG Kim KY Lee H Kim MS Kim WI Cho J Choi IW Nah 2019 α-Fe2O3 anchored on porous N doped carbon derived from green microalgae via spray pyrolysis as anode materials for lithium ion batteries J Ind Eng Chem 69 39 47
XJ Lee HC Ong J Ooi KL Yu TC Tham WH Chen YS Ok 2022 Engineered macroalgal and microalgal adsorbents: Synthesis routes and adsorptive performance on hazardous water contaminants J Hazard Mater 423 126921
C Liu X Duan Q Chen C Chao Z Lu Q Lai M Megharaj 2019 Investigations on pyrolysis of microalgae Diplosphaera sp. MM1 by TG-FTIR and Py-GC/MS: Products and kinetics Bioresour. Technol. 294 122126
L Luo Z Zhang C Li Nishu F He X Zhang J Cai 2021 Insight into master plots method for kinetic analysis of lignocellulosic biomass pyrolysis Energy 233 21194
A Marcilla M Beltrán 1996 Kinetic models for the thermal decomposition of commercial PVC resins and plasticizers studied by thermogravimetric analysis Polym Degrad Stab 53 251 260
SR Naqvi R Tariq M Shahbaz 2021 Recent developments on sewage sludge pyrolysis and its kinetics: Resources recovery, thermogravimetric platforms, and innovative prospects Comput Chem Eng 150 107325
F Qi MM Wright 2020 A DEM modeling of biomass fast pyrolysis in a double auger reactor Int J Heat Mass Transf 150 119308
R Sadegh-Vaziri MU Babler 2018 Modeling of slow pyrolysis of various biomass feedstock in a rotary drum using TGA data Chem Eng Process - Process Intensif 129 95 102
N Sbirrazzuoli 2013 Determination of pre-exponential factors and of the mathematical functions f(α) or G(α) that describe the reaction mechanism in a model-free way Thermochim Acta 564 59 69
GI Senum RT Yang 1977 Rational approximations of the integral of the Arrhenius function J Therm Anal 11 445 447
L Shang J Ahrenfeldt JK Holm LS Bach W Stelte UB Henriksen 2014 Kinetic model for torrefaction of wood chips in a pilot-scale continuous reactor J Anal Appl Pyrolysis 108 109 116
AT Ubando DRT Rivera WH Chen AB Culaba 2019 A comprehensive review of life cycle assessment (LCA) of microalgal and lignocellulosic bioenergy products from thermochemical processes Bioresour Technol 291 121837
G Várhegyi 2019 Empirical Models with Constant and Variable Activation Energy for Biomass Pyrolysis Energ Fuel 33 2348 2358
AK Vuppaladadiyam H Liu M Zhao AF Soomro MZ Memon V Dupont 2019 Thermogravimetric and kinetic analysis to discern synergy during the co-pyrolysis of microalgae and swine manure digestate Biotechnol Biofuels 12 1 1 18
AK Vuppaladadiyam M Zhao MZ Memon AF Soomro 2019 Microalgae as a renewable fuel resource: A comparative study on the thermogravimetric and kinetic behavior of four microalgae Sustain Energ Fuels 3 1283 1296
S Vyazovkin 2021 Determining preexponential factor in model-free kinetic methods: How and why? Molecules 26 11 3077
S Vyazovkin AK Burnham JM Criado LA Pérez-Maqueda C Popescu N Sbirrazzuoli 2011 ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data Thermochim Acta 520 1 19
S Vyazovkin D Dollimore 1996 Linear and nonlinear procedures in isoconversional computations of the activation energy of nonisothermal reactions in solids J Chem Inf Comput Sci 36 42 45
S Wang H Lin B Ru W Sun Y Wang Z Luo 2014 Comparison of the pyrolysis behavior of pyrolytic lignin and milled wood lignin by using TG-FTIR analysis J Anal Appl Pyrolysis 108 78 85
X Wang F Guo Y Li X Yang 2017 Effect of pretreatment on microalgae pyrolysis: Kinetics, biocrude yield and quality, and life cycle assessment Energy Convers Manag 132 161 171
X Wang M Hu W Hu Z Chen S Liu Z Hu B Xiao 2016 Thermogravimetric kinetic study of agricultural residue biomass pyrolysis based on combined kinetics Bioresour Technol 219 510 520
X Wang L Sheng X Yang 2017 Pyrolysis characteristics and pathways of protein, lipid and carbohydrate isolated from microalgae Nannochloropsis sp Bioresour Technol 229 119 125
JE White WJ Catallo BL Legendre 2011 Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies J Anal Appl Pyrolysis 91 1 33
X Xu R Chen R Pan D Zhang 2020 Pyrolysis Kinetics, Thermodynamics, and Volatiles of Representative Pine Wood with Thermogravimetry-Fourier Transform Infrared Analysis Energ Fuel 34 1859 1869
H Yang R Yan H Chen DH Lee C Zheng 2007 Characteristics of hemicellulose, cellulose and lignin pyrolysis Fuel 86 1781 1788
X Zhang Y Li M Wu Y Pang Z Hao M Hu R Qiu Z Chen 2021 Enhanced adsorption of tetracycline by an iron and manganese oxides loaded biochar: Kinetics, mechanism and column adsorption Bioresour Technol 320 124264
H Zheng W Guo S Li Y Chen Q Wu X Feng R Yin SH Ho N Ren JS Chang 2017 Adsorption of p-nitrophenols (PNP) on microalgal biochar: Analysis of high adsorption capacity and mechanism Bioresour Technol 244 1456 1464
Funding
Authors would like to acknowledge the funding from Key Scientific Research Project of Henan Province (No.21A610006), Youth Science Foundation of Henan Normal University (No.5101219170815), and analysis technical supports from Instrumental Analysis & Research Center of Sun Yat-Sen University.
Author information
Authors and Affiliations
Contributions
Xiaoxuan Wang contributed to visualization, data curation, investigation, and data curation; Yanxue Wang contributed to data curation and investigation; Jiaru Guo contributed to formal analysis and validation; Yali Zhao contributed to formal analysis and validation; Xun Wang contributed to software; Xin Zhang contributed to writing—review and editing, supervision, funding acquisition, and project administration; Zhihua Chen contributed to writing—original draft, validation, methodology, and conceptualization.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Wang, Y., Guo, J. et al. Behaviors and non-isothermal kinetics of Chlorella pyrenoidosa fodder pyrolysis by a modified kinetic compensation effects and a parallel two-step reaction model. Biomass Conv. Bioref. 14, 5589–5600 (2024). https://doi.org/10.1007/s13399-022-02723-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02723-7