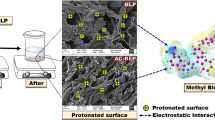
Abstract
Textile dyeing industry sludge was successfully converted into efficient biochar to remove a persistent pharmaceutical pollutant, namely ofloxacin, from an aqueous solution. Raw sludge contained substantial calcium (Ca) attributable to lime as a coagulant in the effluent treatment plant (ETP). It had a reasonable amount of carbon from the cotton yarns dyed in the industry. The raw sludge and prepared biochars were comprehensively examined through characterization techniques like TGA, BET, XRD, XRF, FESEM, EDX, and FTIR. Batch adsorption studies were performed to optimize the operational parameters such as initial ofloxacin concentration, pH of the solution, biochar dose, temperature, and time. The biochar showed the maximum adsorption capacity of 21.6 mg g−1 at optimum conditions (C0 = 30 mg L−1, T = 25 ± 2 °C, pH = 6 ± 0.2, dose = 5 g L−1, time = 240 min). The pseudo 2nd-order kinetics well explained the experimental adsorption data with regression coefficient in the range 0.944 ≤ R2 ≤ 0.998 and sum of square of error (SSE) varying in the range 0.3 ≤ SSE ≤ 29.5. Langmuir isotherm closely describes the ofloxacin adsorption with 0.877 ≤ R2 ≤ 0.965 and 5.1 ≤ SSE ≤ 12.4. The thermal regeneration study of biochar was also carried out, and the removal efficiency of ≈73% was obtained in the first cycle while it trends to decrease to ≈59% after five consecutive cycles. The cost analysis showed that biochar could be prepared for ≈$0.305 (₹22.8) per kg. Hence, biochar is reasonably economical and could be used for large-scale applications.
Graphical abstract







Similar content being viewed by others
References
Wong S, Yaćcob NAN, Ngadi N, Hassan O, Inuwa IM (2018) From pollutant to solution of wastewater pollution: synthesis of activated carbon from textile sludge for dye adsorption. Chinese J Chem Eng 26:870–878. https://doi.org/10.1016/j.cjche.2017.07.015
Kumar P, Samuchiwal S, Malik A (2020) Anaerobic digestion of textile industries wastes for biogas production. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00601-8
Tang X, Chen X, He Y, Evrendilek F, Chen Z, Liu J (2022) Co-pyrolytic performances, mechanisms, gases, oils, and chars of textile dyeing sludge and waste shared bike tires under varying conditions. Chem Eng J 428:131053. https://doi.org/10.1016/j.cej.2021.131053
Sonai GG, de Souza Smagu, de Oliveira D, de Souza AAU (2016) The application of textile sludge adsorbents for the removal of Reactive Red 2 dye. J Environ Manage 168:149–156. https://doi.org/10.1016/j.jenvman.2015.12.003
Man X, Ning XA, Zou H, Liang J, Sun J, Lu X, Sun J (2018) Removal of polycyclic aromatic hydrocarbons (PAHs) from textile dyeing sludge by ultrasound combined zero-valent iron/EDTA/air system. Chemosphere 191:839–847. https://doi.org/10.1016/j.chemosphere.2017.10.043
Darwesh OM, Abd El-Latief AH, Abuarab ME, Kasem MA (2021) Enhancing the efficiency of some agricultural wastes as low-cost absorbents to remove textile dyes from their contaminated solutions. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01142-w
Hui TS, Zaini MAA (2020) Textile sludge–sawdust chemically produced activated carbon: equilibrium and dynamics studies of malachite green adsorption. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00955-z
Cai H, Liu J, Kuo J, Xie W, Evrendilek F, Zhang G (2021) Ash-to-emission pollution controls on co-combustion of textile dyeing sludge and waste tea. Sci Total Environ 794:148667. https://doi.org/10.1016/j.scitotenv.2021.148667
Dondapati SKS, Ravikumar TS, Guthi VR, Ade AD, Deekala RS, Kondagunta N (2018) A comparative study of general waste management practices in a campus of a medical university located in the purview of a municipal corporation of a South Indian state. Int J Community Med Public Heal 5:5115. https://doi.org/10.18203/2394-6040.ijcmph20184721
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80. https://doi.org/10.1016/j.jhazmat.2009.12.047
Beshah DA, Tiruye GA, Mekonnen YS (2021) Characterization and recycling of textile sludge for energy-efficient brick production in Ethiopia. Environ Sci Pollut Res 28:16272–16281. https://doi.org/10.1007/s11356-020-11878-7
Ding R, Zhang P, Seredych M, Bandosz TJ (2012) Removal of antibiotics from water using sewage sludge and waste oil sludge-derived adsorbents. Water Res 46:4081–4090. https://doi.org/10.1016/j.watres.2012.05.013
Smith KM, Fowler GD, Pullket S, Graham NJD (2009) Sewage sludge-based adsorbents: a review of their production, properties and use in water treatment applications. Water Res 43:2569–2594. https://doi.org/10.1016/j.watres.2009.02.038
Leal TW, Lourenco LA, Scheibe AS, De Souza SMGU, De Souza AAU (2018) Textile wastewater treatment using low-cost adsorbent aiming the water reuse in dyeing process. J Environ Chem Eng 6:2705–2712. https://doi.org/10.1016/j.jece.2018.04.008
Sohaimi KSA, Ngadi N, Mat H, Inuwa IM, Wong S (2017) Synthesis, characterization and application of textile sludge biochars for oil removal. J Environ Chem Eng 5:1415–1422. https://doi.org/10.1016/j.jece.2017.02.002
Sharma P, Kumar N, Chauhan R, Singh V, Srivastava VC, Bhatnagar R (2020) Growth of hierarchical ZnO nano flower on large functionalized rGO sheet for superior photocatalytic mineralization of antibiotic. Chem Eng J 392:123746. https://doi.org/10.1016/j.cej.2019.123746
Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, Goossens H, Laxminarayan R (2018) Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA 115:E3463–E3470. https://doi.org/10.1073/pnas.1717295115
Balakrishna K, Rath A, Praveenkumarreddy Y, Guruge KS, Subedi B (2017) A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol Environ Saf 137:113–120. https://doi.org/10.1016/j.ecoenv.2016.11.014
Chen YR, Guo XP, Feng JN, Lu DP, Niu ZS, Tou FY, Hou LJ, Liu M, Yang Y (2019) Impact of ZnO nanoparticles on the antibiotic resistance genes (ARGs) in estuarine water: ARG variations and their association with the microbial community. Environ Sci Nano 6:2405–2419. https://doi.org/10.1039/c9en00338j
He LY, Liu YS, Su HC, Zhao JL, Liu SS, Chen J, Liu WR, Ying GG (2014) Dissemination of antibiotic resistance genes in representative broiler feedlots environments: identification of indicator ARGs and correlations with environmental variables. Environ Sci Technol 48:13120–13129. https://doi.org/10.1021/es5041267
Kaur R, Kushwaha JP, Singh N (2019) Electro-catalytic oxidation of ofloxacin antibiotic in continuous reactor: evaluation, transformation products and pathway. J Electrochem Soc 166:H250–H261. https://doi.org/10.1149/2.1281906jes
Peng H, Pan B, Wu M, Liu Y, Zhang D, Xing B (2012) Adsorption of ofloxacin and norfloxacin on carbon nanotubes: hydrophobicity- and structure-controlled process. J Hazard Mater 233–234:89–96. https://doi.org/10.1016/j.jhazmat.2012.06.058
Putra EK, Pranowo R, Sunarso J, Indraswati N, Ismadji S (2009) Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: mechanisms, isotherms and kinetics. Water Res 43:2419–2430. https://doi.org/10.1016/j.watres.2009.02.039
Patidar R, Srivastava VC (2021) Ultrasound-assisted electrochemical treatment of cosmetic industry wastewater: mechanistic and detoxification analysis. J Hazard Mater 126842. https://doi.org/10.1016/j.jhazmat.2021.126842
Patidar R, Srivastava VC (2021) Ultrasound-induced intensification of electrochemical treatment of bulk drug pharmaceutical wastewater. ACS ES&T Water 1:1941–1954. https://doi.org/10.1021/acsestwater.1c00159
Singh V, Srivastava VC (2020) Self-engineered iron oxide nanoparticle incorporated on mesoporous biochar derived from textile mill sludge for the removal of an emerging pharmaceutical pollutant. Environ Pollut 259:113822. https://doi.org/10.1016/j.envpol.2019.113822
Sharma A, Mohanty B (2020) Non-isothermal TG/DTG-FTIR kinetic study for devolatilization of Dalbergia sissoo wood under nitrogen atmosphere. J Therm Anal Calorim. https://doi.org/10.1007/s10973-020-09978-0
Ding Z, Liu J, Chen H, Huang S, Evrendilek F, He Y, Zheng L (2021) Co-pyrolysis performances, synergistic mechanisms, and products of textile dyeing sludge and medical plastic wastes. Sci Total Environ 149397. https://doi.org/10.1016/j.scitotenv.2021.149397
Singh V, Chakravarthi MH, Srivastava VC (2020) Chemically modified biochar derived from effluent treatment plant sludge of a distillery for the removal of an emerging pollutant, tetracycline, from aqueous solution. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00683-4
Lanzetta M, Blasi CD (1998) Pyrolysis kinetics of wheat and corn straw. J Anal Appl Pyrolysis 44:181–192. https://doi.org/10.1016/S0165-2370(97)00079-X
Wang D, Xiao R, Zhang H, He G (2010) Comparison of catalytic pyrolysis of biomass with MCM-41 and CaO catalysts by using TGA-FTIR analysis. J Anal Appl Pyrolysis 89:171–177. https://doi.org/10.1016/j.jaap.2010.07.008
Méndez A, Barriga S, Fidalgo JM, Gascó G (2009) Adsorbent materials from paper industry waste materials and their use in Cu(II) removal from water. J Hazard Mater 165:736–743. https://doi.org/10.1016/j.jhazmat.2008.10.055
Yao Y, Lian C, Wu G, Hu Y, Wei F, Yu M, Wang S (2017) Synthesis of “sea urchin”-like carbon nanotubes/porous carbon superstructures derived from waste biomass for treatment of various contaminants. Appl Catal B Environ 219:563–571. https://doi.org/10.1016/j.apcatb.2017.07.064
Luo X, Song X, Cao Y, Song L, Bu X (2020) Investigation of calcium carbonate synthesized by steamed ammonia liquid waste without use of additives. RSC Adv 10:7976–7986. https://doi.org/10.1039/c9ra10460g
Liu T, Guo Y, Peng N, Lang Q, Xia Y, Gai C, Liu Z (2017) Nitrogen transformation among char, tar and gas during pyrolysis of sewage sludge and corresponding hydrochar. J Anal Appl Pyrolysis 126:298–306. https://doi.org/10.1016/j.jaap.2017.05.017
Wankhede ME, Haram SK (2003) Synthesis and characterization of CD-DMSO complex capped CdS nanoparticles. Chem Mater 15:1296–1301. https://doi.org/10.1021/cm020761w
Ojeda JJ, Romero-Gonzalez ME, Bachmann RT, Edyvean RG, Banwart SA (2008) Characterization of the cell surface and cell wall chemistry of drinking water bacteria by combining XPS, FTIR spectroscopy, modeling, and potentiometric titrations. Langmuir 24:4032–4040. https://doi.org/10.1021/la702284b
Tomer R, Biswas P (2020) Dehydration of glucose/fructose to 5-hydroxymethylfurfural (5-HMF) over an easily recyclable sulfated titania (SO42−/TiO2) catalyst. New Journal of Chemistry. 44:20,734-20,750. https://doi.org/10.1039/D0NJ04151C
Park HR, Kim TH, Bark KM (2002) Physicochemical properties of quinolone antibiotics in various environments. Eur J Med Chem 37:443–460. https://doi.org/10.1016/S0223-5234(02)01361-2
Jing XR, Wang YY, Liu WJ, Wang YK, Jiang H (2014) Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem Eng J 248:168–174. https://doi.org/10.1016/j.cej.2014.03.006
Kong Q, He X, Shu L, Miao M, sheng, (2017) Ofloxacin adsorption by activated carbon derived from luffa sponge: kinetic, isotherm, and thermodynamic analyses. Process Saf Environ Prot 112:254–264. https://doi.org/10.1016/j.psep.2017.05.011
Jaria G, Calisto V, Gil MV, Otero M, Esteves VI (2015) Removal of fluoxetine from water by adsorbent materials produced from paper mill sludge. J Colloid Interface Sci 448:32–40. https://doi.org/10.1016/j.jcis.2015.02.002
Wu Z, Zhong H, Yuan X, Wang H, Wang L, Chen X, Zeng G, Wu Y (2014) Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res 67:330–344. https://doi.org/10.1016/j.watres.2014.09.026
Singh P, Singh R, Borthakur A, Madhav S, Singh VK, Tiwary D, Srivastava VC, Mishra PK (2018) Exploring temple floral refuse for biochar production as a closed loop perspective for environmental management. Waste Manag 77:78–86. https://doi.org/10.1016/j.wasman.2018.04.041
Rauthula MS, Srivastava VC (2011) Studies on adsorption/desorption of nitrobenzene and humic acid onto/from activated carbon. Chem Eng J 168:35–43. https://doi.org/10.1016/j.cej.2010.12.026
Chakraborty P, Singh SD, Gorai I, Singh D, Rahman WU, Halder G (2020) Explication of physically and chemically treated date stone biochar for sorptive remotion of ibuprofen from aqueous solution. J Water Process Eng. 33:101022. https://doi.org/10.1016/j.jwpe.2019.101022
Orlandi G, Cavasotto J, Machado FRS, Colpani GL, Magro JD, Dalcanton F, Mello JMM, Fiori MA (2017) An adsorbent with a high adsorption capacity obtained from the cellulose sludge of industrial residues. Chemosphere 169:171–180. https://doi.org/10.1016/j.chemosphere.2016.11.071
Banerjee S, Mukherjee S, LaminKa-ot A, Joshi SR, Mandal T, Halder G (2016) Biosorptive uptake of Fe2+, Cu2+ and As5+ by activated biochar derived from Colocasia esculenta: isotherm, kinetics, thermodynamics, and cost estimation. J Adv Res 7:597–610. https://doi.org/10.1016/j.jare.2016.06.002
Mondal S, Bobde K, Aikat K, Halder G (2016) Biosorptive uptake of ibuprofen by steam activated biochar derived from mung bean husk: equilibrium, kinetics, thermodynamics, modeling and eco-toxicological studies. J Environ Manage 182:581–594. https://doi.org/10.1016/j.jenvman.2016.08.018
Li W, Yue Q, Tu P, Ma Z, Gao B, Li J, Xu X (2011) Adsorption characteristics of dyes in columns of activated carbon prepared from paper mill sewage sludge. Chem Eng J 178:197–203. https://doi.org/10.1016/j.cej.2011.10.049
Singh V, Srivastava VC (2022) Hazardous maize processing industrial sludge: thermo-kinetic assessment and sulfur recovery by evaporation-condensation technique. J Hazard Mater 424:127477. https://doi.org/10.1016/j.jhazmat.2021.127477
Funding
Financial support was provided by the Indian Institute of Technology Roorkee, Uttarakhand, India, to purchase the TG-FTIR instrument from the SMILE grant.
Author information
Authors and Affiliations
Contributions
Vikash Singh: conceptualization, methodology, data curation, writing—original draft; Vimal Chandra Srivastava: supervision, conceptualization, writing — review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, V., Srivastava, V.C. Transformation of textile dyeing industrial sludge into economical biochar for sorption of ofloxacin: equilibrium, kinetic, and cost analysis. Biomass Conv. Bioref. 14, 1881–1893 (2024). https://doi.org/10.1007/s13399-022-02554-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02554-6