Abstract
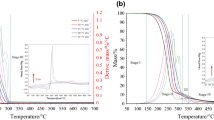
According to the United States Energy Information Administration (US EIA), the annual pure biodiesel production in the USA was 6.88 million cubic meters in 2020. Similarly, significant biodiesel production was reported across the world with countries like Brazil, Germany, and Indonesia estimated to have produced 5.9, 3.2, and 6.2 million cubic meters in 2019. This enormous biodiesel production, which is widespread globally, has led to huge amount of waste in the form of glycerol as a product of transesterification. It was estimated that crude glycerol production was 189.27 million cubic meters in 2021. Thermochemical conversion processes, such as combustion, gasification, and pyrolysis, are viable ways to utilize the waste glycerol. In this study, the thermogravimetric analyzer was used to investigate and compare the characteristics of crude and pure glycerol combustion. In particular, the kinetic and thermodynamic parameters as well as the ignition and burnout temperatures were evaluated for the two fuels. The glycerol samples were subjected to a temperature range of 50–700 °C under dissimilar heating rates, i.e., 5, 10, 15, and 20 °C/min. Results of the thermal decomposition process indicate that while there is a single stage in pure glycerol, the crude glycerol is characterized with three decomposition stages. In addition, the experimental results showed that the main combustion process in both samples occurred at about 150–(325 ± 25) °C. The effect of heating rate on TG and DTG curves showed that at higher heating rates, the degradation curves shifts to higher temperature values. The crude glycerol ignition temperature is in the range of 195–218 °C compared to 180–212 °C for pure glycerol, and their burnout temperatures were 463–508 °C and 238–276 °C, respectively. The activation energies were evaluated with Kissinger method and found to be 75 kJ/mol for the devolatilization event in the crude glycerol and 79.6 kJ/mol for the pure glycerol.
Graphical abstract












Similar content being viewed by others
Data availability
Any needed data presented in this work is available and can be obtained by emailing the corresponding author Isam.Janajreh@ku.ac.ae who is open to share them accordingly.
References
EIA US (2020) Monthly biodiesel production report. Washington, Independent Statics & Analysis. U.S. Department of Energy Washington, DC 20585. www.eia.gov
da Silva Ruy AD, Ferreira ALF, Bresciani AÉ, de Brito Alves RM, Pontes LAM (2020) Market prospecting and assessment of the economic potential of glycerol from biodiesel. Biotechnol Appl Biomass. https://doi.org/10.5772/intechopen.93965
Tiseo I. Global biodiesel production by country 2019 [Internet]. Available from: https://www.statista.com/statistics/271472/biodiesel-production-in-selected-countries/ . Accessed date: 07 Jul 2020
Gabriel KCP, Barros AAC, Correia MJN (2015) Study of molar ratio in biodiesel production from palm oil. International Association for Management of Technology, IAMOT Conference, Cape Town, South Africa, pp. 434–442
Colombo K, Ender L, Chivanga Barros AA (2017) The study of biodiesel production using CaO as a heterogeneous catalytic reaction Egypt. J. Petrol. 26(2):341–349
Khazayialiabad A, Iranshahi D (2021) Inherent CO2 capture and H2 production enhancement in a new glycerol steam reformer coupled with chemical looping combustion. Am Chem Soc Energy Fuels 35(6):5049–5063. https://doi.org/10.1021/acs.energyfuels.0c02664
Giuseppe B, Adolfo I, Aimaro S, Angelo B (2017) Glycerol production and transformation: a critical review with particular emphasis on glycerol reforming reaction for producing hydrogen in conventional and membrane reactors. Membrane, MDPI, 1–31. https://doi.org/10.3390/membranes7020017
Haas MJ, Mcaloon AJ, Yee WC, Foglia TA (2006) A process model to estimate biodiesel production costs. Bioresour Technol 94:671–678
Chilakamarry CR, Sakinah AM, Zularisam AW, Pandey A (2021) Glycerol waste to value added products and its potential applications. Systems Microbiology and Biomanufacturing 1(4):378–396
Lam MK, Lee KT, Mohamed AR (2010) Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: a review. Biotechnol Adv 28(4):500–518
Janajreh I, ElSamad T, Hussain MN (2018) Intensification of transesterification via sonication numerical simulation and sensitivity study. Appl Energy 185:2151–2159
Noureddini H, Zhu D (1997) Kinetics of transesterification of soybean oil. Journal of the American Oil Chemists’ Society 74(11):1457–1463
Fountoulakis MS, Manios T (2009) Enhanced methane and hydrogen production from municipal solid waste and agro-industrial by-products co-digested with crude glycerol. Biores Technol 100(12):3043–3047
Zhang M, Wu H (2015) Effect of major impurities in crude glycerol on solubility and properties of glycerol/methanol/bio-oil blends. Fuel 159:118–127
Mangayil R, Karp M, Santala V (2012) Bioconversion of crude glycerol from biodiesel production to hydrogen. Int J Hydrogen Energy 37(17):12198
Varrone C, Liberatore R, Crescenzi T, Izzo G, Wang A (2013) The valorization of glycerol: economic assessment of an innovative process for the bioconversion of crude glycerol into ethanol and hydrogen. Appl Energy 105:349–357
Luo X, Ge X, Cui S, Li Y (2016) Value-added processing of crude glycerol into chemicals and polymers. Biores Technol 215:144–154
Chongkhong S, Tongurai C, Chetpattananondh P (2009) Continuous esterification for biodiesel production from palm fatty acid distillate using economical process. Renew Energy 34(4):1059–1063
Skoulou VK, Manara P, Zabaniotou AA (2012) H2 enriched fuels from co-pyrolysis of crude glycerol with biomass. J Anal Appl Pyrolysis 97:198–204
Skoulou VK, Zabaniotou AA (2013) Co-gasification of crude glycerol with lignocellulosic biomass for enhanced syngas production. J Anal Appl Pyrolysis 99:110–116
Dou B et al (2010) Steam reforming of crude glycerol with in situ CO2 sorption. Bioresource Technol 101(7):2436–2442
Bohon MD, Metzger BA, Linak WP, King CJ, Roberts WL (2011) Glycerol combustion and emissions. Proc Combust Inst 33(2):2717–2724
Steinmetz SA, Herrington JS, Winterrowd CK, Roberts WL, Wendt JOL, Linak WP (2013) Crude glycerol combustion: particulate, acrolein, and other volatile organic emissions. Proc Combust Inst 34(2):2749–2757
Bartocci P et al (2017) Pyrolysis of pellets made with biomass and glycerol: kinetic analysis and evolved gas analysis. Biomass Bioenerg 97:11–19
Yuan X et al (2016) Pyrolysis and combustion kinetics of glycerol-in-diesel hybrid fuel using thermogravimetric analysis. Fuel 182:502–508
Crnkovic PM, Koch C, Ávila I, Mortari DA, Cordoba AM, Moreira dos Santos A (2012) Determination of the activation energies of beef tallow and crude glycerin combustion using thermogravimetry. Biomass Bioenerg 44:8–16
Castelló ML, Dweck J, Aranda DAG (2011) Kinetic study of thermal processing of glycerol by thermogravimetry. J Therm Anal Calorim 105(3):737–746
Almazrouei M, Janajreh I (2020) Model-fitting approach to kinetic analysis of non-isothermal pyrolysis of pure and crude glycerol. Renew Energy 145:1693–1708
Almazrouei M, Janajreh I (2019) Thermogravimetric study of the combustion characteristics of biodiesel and petroleum diesel. J Therm Anal Calorim 136.2:925–935
Dou B, Dupont V, Williams PT, Chen H, Ding Y (2009) Thermogravimetric kinetics of crude glycerol. Biores Technol 100(9):2613–2620
Lu J-J, Chen W-H (2015) Investigation on the ignition and burnout temperatures of bamboo and sugarcane bagasse by thermogravimetric analysis. Appl Energy 160:49–57
Huang L et al (2016) Thermodynamics and kinetics parameters of co-combustion between sewage sludge and water hyacinth in CO2/O2 atmosphere as biomass to solid biofuel. Biores Technol 218:631–642
Niu S-L, Han K-H, Lu C-M (2011) Characteristic of coal combustion in oxygen/carbon dioxide atmosphere and nitric oxide release during this process. Energy Convers Manage 52(1):532–537
Xiu S, Rojanala HK, Shahbazi A, Fini EH, Wang L (2012) Pyrolysis and combustion characteristics of Bio-oil from swine manure. J Therm Anal Calorim 107(2):823–829
Sahu SG, Sarkar P, Chakraborty N, Adak AK (2010) Thermogravimetric assessment of combustion characteristics of blends of a coal with different biomass chars. Fuel Process Technol 91(3):369–378
Ahn S, Choi G, Kim D (2014) The effect of wood biomass blending with pulverized coal on combustion characteristics under oxy-fuel condition. Biomass Bioenerg 71:144–154
Zhang B et al (2017) Investigation on the ignition, thermal acceleration and characteristic temperatures of coal char combustion. Appl Therm Eng 113:1303–1312
Blaine RL, Kissinger HE (2012) Homer Kissinger and the Kissinger equation. Thermochim Acta 540:1
Kaur R, Gera P, Jha MK, Bhaskar T (2018) Pyrolysis kinetics and thermodynamic parameters of castor (Ricinus communis) residue using thermogravimetric analysis. Bioresour Technol 250:422–428
Yuan X, He T, Cao H, Yuan Q (2017) Cattle manure pyrolysis process: kinetic and thermodynamic analysis with isoconversional methods. Renew Energy 107:489–496
Niu S, Chen M, Li Y, Xue F (2016) Evaluation on the oxy-fuel combustion behavior of dried sewage sludge. Fuel 178:129–138
Gao W, Zhang M, Wu H (2017) Ignition temperatures of various bio-oil based fuel blends and slurry fuels. Fuel 207:240–243
Xu Y, Chen B (2013) Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Biores Technol 146:485–493
Khan AS et al (2016) Kinetics and thermodynamic parameters of ionic liquid pretreated rubber wood biomass. J Mol Liq 223:754–762
Bohon MD, Metzger BA, Linak WP, King CJ, Roberts WL (2011) Glycerol combustion and emissions. Proc Combust Inst 33(2):2717–2724
Funding
We acknowledge the financial support received by the Khalifa University under Award No. RC2-2018–009.
Author information
Authors and Affiliations
Contributions
The contribution of the work is as follows: Manar Almazrouei: experimental and data analysis and interpretation, methodology, writing, validation, reviewing, editing, and software development. Idowu Adeyemi: methodology, data curation and analysis, writing, reviewing and editing. Isam Janajreh: conceptualization of the original draft preparation, data analysis and interpretation, methodology, investigation and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The decomposition of pure glycerol was in a single stage, but crude glycerol was in three stages
• Ignition temperature of crude was within 195–218 °C and pure glycerol within 180–212 °C
• Burnout temperature of crude was 463–508 °C and for pure glycerol was 238–276 °C
• Devolatilization activation energies were 75 kJ/mol for crude and 79.6 kJ/mol for pure glycerol
• Ignition index of pure glycerol performed better than the waste glycerol at all heating rates
Rights and permissions
About this article
Cite this article
Almazrouei, M., Adeyemi, I. & Janajreh, I. Thermogravimetric assessment of the thermal degradation during combustion of crude and pure glycerol. Biomass Conv. Bioref. 12, 4403–4417 (2022). https://doi.org/10.1007/s13399-022-02526-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02526-w