Abstract
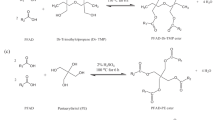
Palm fatty acid distillate is a processing by-product resulting from physical refining of crude palm oil products. Producing renewable fuels and biodegradable ester-based biolubricants using saturated palm fatty acid distillate (SFA) is one of environmentally friendly and an efficient way to reuse such by-product for the production of feasible high-end products. This study was carried out to synthesize and determine the optimal conditions for the esterification of Malaysian SFA with high degree polyhydric alcohols (trimethylolpropane, TMP; di-trimethylolpropane, Di-TMP; pentaerythritol, PE; and di-pentaerythritol, Di-PE) in the presence of sulfuric acid as catalyst. The esterification reaction parameters involved such as amount of catalyst, effect of reaction temperature (°C) and time (h), and effect of different alcohols, and molar ratio used was investigated. The results showed that the ester yield percentages varied with the corresponding reaction parameters and chemical structure of the alcohol used. The resultant esters with small-branched hydrocarbon chain alcohol produced the highest yield percentage for the SFA-TMP tri-ester (89 ± 3%) followed by the SFA-Di-TMP tetra-ester (87 ± 4%), SFA-PE tetra-ester (83 ± 5%), and SFA-Di-PE hexa-ester (77 ± 4%), respectively. The results showed the reaction temperature has more significant effect on the esterification reaction yield as compared to other parameters. The lubrication properties of the synthesized polyol esters indicated their appropriateness to be used as grease and bearing lubricant application.
















Similar content being viewed by others
References
Tulashie SK, Kotoka F (2020) The potential of castor, palm kernel, and coconut oils as biolubricant base oil via chemical modification and formulation. Therm Sci Eng Prog 16:100480. https://doi.org/10.1016/j.tsep.2020.100480
Ahmed WA, Salih N, Salimon J (2021) Lubricity, tribological and rheological properties of green ester oil prepared from bio-based azelaic acid. Asian J Chem 33:1363–1369. https://doi.org/10.14233/ajchem.2021.23209
Salih N, Salimon J (2022) A review on new trends, challenges and prospects of ecofriendly friendly green food-grade biolubricants. Biointerface Res Appl Chem 12:1185–1207. https://doi.org/10.33263/BRIAC121.11851207
Afifah AN, Syahrullail S, Azlee NIW, Sidik NAC, Yahya WJ, Abd Rahim E (2019) Biolubricant production from palm stearin through enzymatic transesterification method. Biochem Eng J 148:178–184. https://doi.org/10.1016/j.bej.2019.05.009
Sapawe N, Hanafi MF, Samion S (2019) The use of palm oil as new alternative biolubricant for improving anti-friction and anti-wear properties. Mater today: proceed 19:1126–1135. https://doi.org/10.1016/j.matpr.2019.11.005
Salih N, Salimon J (2021) A Review on eco-friendly green biolubricants from renewable and sustainable plant oil sources. Biointerface Res Appl Chem 11:13303–13327. https://doi.org/10.33263/BRIAC115.1330313327
Calero J, Luna D, Sancho ED, Luna C, Bautista FM, Romero AA, Posadillo A, Berbel J, Verdugo-Escamilla C (2015) An overview on glycerol-free processes for the production of renewable liquid biofuels, applicable in diesel engines. Renew Sustain Energy Rev 42:1437–1452. https://doi.org/10.1016/j.rser.2014.11.007
Nor NM, Salih N, Salimon J (2021) Optimization of the ring opening of epoxidized palm oil using D-optimal design. Asian J Chem 33:67–75. https://doi.org/10.14233/ajchem.2021.22938
Mba OI, Dumont MJ, Ngadi M (2015) Palm oil: processing, characterization and utilization in the food industry - a review. Food Biosci 10:26–41. https://doi.org/10.1016/j.fbio.2015.01.003
Teng S, Khong KW, Ha NC (2020) Palm oil and its environmental impacts: a big data analytics study. J Clean Prod 274:122901. https://doi.org/10.1016/j.jclepro.2020.122901
Japir AA, Salih N, Salimon J (2021) Synthesis and characterization of biodegradable palm palmitic acid based bioplastic. Turkish J Chem 45:585–599. https://doi.org/10.3906/kim-2011-31
Koushki M, Nahidi M, Cheraghali F (2015) Physico-chemical properties, fatty acid profile and nutrition in palm oil. Arch Adv Biosci 6:117–134. https://doi.org/10.22037/jps.v6i3.9772
Ludin NA, Bakri MAM, Kamaruddin N, Sopian K, Deraman MS, Hamid NH, Asim N, Othman MY (2014) Malaysian oil palm plantation sector: exploiting renewable energy toward sustainability production. J Clean Prod 65:9–15. https://doi.org/10.1016/j.jclepro.2013.11.063
Mannu A, Ferro M, Dugoni GC, Garroni S, Taras A, Mele A (2020) Response surface analysis of density and flash point in recycled waste cooking oils. Chem Data Collect 25:100329. https://doi.org/10.1016/j.cdc.2019.100329
Zulkifli NWM, Azman SSN, Kalam MA, Masjuki HH, Yunus R, Gulzar M (2016) Lubricity of bio-based lubricant derived from different chemically modified fatty acid methyl ester. Tribol Int 93:555–562. https://doi.org/10.1016/j.triboint.2015.03.024
Ping BTY, Yusof M (2009) Characteristics and properties of fatty acid distillates from palm oil. Oil palm bull 59:5–11
Metre AV, Nath K (2015) Super phosphoric acid catalyzed esterification of palm fatty acid distillate for biodiesel production: physicochemical parameters and kinetics. Polish J Chem Technol 17:88–96. https://doi.org/10.1515/pjct-2015-0013
Jumaah MA, Yousif MFM, Salimon J, Murad B (2019) Separation of saturated and unsaturated fatty acids of palm fatty acid distilled via low-temperature methanol crystallization. Malaysian J Chem 21:8–16
Baharudin KB, Taufiq-Yap YH, Hunns J, Isaacs M, Wilson K, Derawi D (2019) Mesoporous NiO/Al-SBA-15 catalysts for solvent-free deoxygenation of palm fatty acid distillate. Micropor Mesopor Mat 276:13–22. https://doi.org/10.1016/j.micromeso.2018.09.014
Haraldsson G (1984) Separation of saturated/unsaturated fatty acids. J Am Oil Chem Soc 61:219–222. https://doi.org/10.1007/BF02678772
Bahadi M, Salih N, Salimon J (2021) D-Optimal design optimization for the separation of oleic acid from Malaysian high free fatty acid crude palm oil fatty acids mixture using urea complex fractionation. Appl Sci Eng Prog 14:175–186. https://doi.org/10.14416/j.asep.2021.03.004
Machado NT, Brunner G (1997) Separation of saturated and unsaturated fatty acids from palm fatty acids distillates in continuous multistage counter current columns with supercritical carbon dioxide as solvent: a process design methodology. Ciênc Tecnol Aliment 17:361–370
Qiwen Y, Guoming Y, Haijun L (2021) Extraction and separation of unsaturated fatty acids from sunflower oil. IOP Conf Series: Earth Environ Sci 680:012063. https://doi.org/10.1088/1755-1315/680/1/012063
Nor NM, Derawi D, Salimon J (2019) Esterification and evaluation of palm oil as biolubricant base stock. Malaysian J Chem 21:28–35
Nowicki J, Stańczyk D, Drabik J, Mosio-Mosiewski J, Woszczyński P, Warzała M (2016) Synthesis of fatty acid esters of selected higher polyols over homogeneous metallic catalysts. J Am Oil Chem Soc 93:973–981. https://doi.org/10.1007/s11746-016-2840-7
Pavia DL, Lampman GM, Kriz GS, Vyvyan JR (2015) Introduction to spectroscopy, 5th edn. Cengage Learning Inc, USA
Polansky R, Prosr P, Vik R, Moravcova D, Pihera J (2017) Comparison of the mineral oil lifetime estimates obtained by differential scanning calorimetry, infrared spectroscopy, and dielectric dissipation factor measurements. Thermochim Acta 647:86–93. https://doi.org/10.1016/j.tca.2016.12.002
Alexandri E, Ahmed R, Siddiqui H, Choudhary MI, Tsiafoulis CG, Gerothanassis IP (2017) High resolution NMR spectroscopy as a structural and analytical tool for unsaturated lipids in solution. Molecules 22:1663. https://doi.org/10.3390/molecules22101663
Mortier RM, Fox MF, Orszulik S (2010) Chemistry and technology of lubricants, 3rd edn. Springer, New York
Lye YN, Salih N, Salimon J (2021) Optimization of partial epoxidation on Jatropha curcas oil based methyl linoleate using urea-hydrogen peroxide and methyltrioxorhenium catalyst. Appl Sci Eng Prog 14:89–99. https://doi.org/10.14416/j.asep.2020.12.006
Jumaah MA, Salih N, Salimon J (2021) Optimization for esterification of palm fatty acid distillate based biolubricant using D-optimal design. Iran J Chem Chem Eng. https://doi.org/10.30492/IJCCE.2021.521586.4481
Samidin S, Salih N, Salimon J (2021) Synthesis and characterization of trimethylolpropane based esters as green biolubricant basestock. Biointerface Res Appl Chem 11:13638–13651. https://doi.org/10.33263/BRIAC115.1363813651
Nor NM, Salih N, Salimon J (2021) Chemically modified Jatropha curcas oil for biolubricant applications. Hem Ind 75:117–128. https://doi.org/10.2298/HEMIND200809009N
Jumaah MA, Salih N, Salimon J (2022) Optimization process for the synthesis of polyol-oleates from Malaysian unsaturated palm fatty acid distillate. Iran J Chem Chem Eng. https://doi.org/10.30492/IJCCE.2022.526491.4617
Ocholi O, Menkiti M, Auta M, Ezemagu I (2018) Optimization of the operating parameters for the extractive synthesis of biolubricant from sesame seed oil via response surface methodology. Egypt J Pet 27:265–275. https://doi.org/10.1016/j.ejpe.2017.04.001
Owuna FJ, Dabai MU, Sokoto MA, Dangoggo SM, Bagudo BU, Birnin-Yauri UA, Hassan LG, Sada I, Abubakar AL, Jibrin MS (2020) Chemical modification of vegetable oils for the production of biolubricants using trimethylolpropane: a review. Egypt J Pet 29:75–82. https://doi.org/10.1016/j.ejpe.2019.11.004
Totten GE, Westbrook SR, Shah RJ (2003) Fuels and lubricants handbook: technology, properties, performance, and testing New York, USA.
Cermak SC, Brandon KB, Isbell TA (2006) Synthesis and physical properties of estolides from lesquerella and castor fatty acid esters. Ind Crops Prod 23:54–64. https://doi.org/10.1016/j.indcrop.2005.04.001
Verma P, Dwivedi G, Behura AK, Patel DK, Verma TN, Pugazhendhi A (2020) Experimental investigation of diesel engine fuelled with different alkyl esters of karanja oil. Fuel 275:117920. https://doi.org/10.1016/j.fuel.2020.117920
Warasanam S, Pengprecha S (2012) Synthesis of pour point depressant from dicarboxylic acid for biodiesel. Proceed Inter Conf Chem Process Environ Issues, Singapore 15–16:183–186
Encinar JM, Nogales-Delgado S, Sánchez N, González JF (2020) Biolubricants from rapeseed and castor oil transesterification by using titanium isopropoxide as a catalyst: production and characterization. Catalysts 10:366. https://doi.org/10.3390/catal10040366
Chan CH, Tang SW, Mohd NK, Lim WH, Yeong SK, Idris Z (2018) Tribological behavior of biolubricant base stocks and additives. Renew Sust Energy Rev 93:145–157. https://doi.org/10.1016/j.rser.2018.05.024
Arbain NH, Salimon J, Salih N, Ahmed WA (2022) Optimization for epoxidation of Malaysian Jatropha curcas oil based trimethylolpropane ester biolubricant. Appl Sci Eng Prog. https://doi.org/10.14416/j.asep.2021.10.009
Funding
This project received funding from Universiti Kebangsaan Malaysia under the research grant no. GUP-2016–058 and Sime Darby Plantation Berhad through grant no. ST-2014–019.
Author information
Authors and Affiliations
Contributions
Majd Ahmed Jumaah: investigation and methodology. Firas Layth Khaleel: formal data analysis and data curation. Nadia Salih: visualization, writing—original draft, and writing—review and editing. Jumat Salimon: conceptualization, funding acquisition, resources, project administration, supervision, validation, and writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jumaah, M.A., Khaleel, F.L., Salih, N. et al. Synthesis of tri, tetra, and hexa-palmitate polyol esters from Malaysian saturated palm fatty acid distillate for biolubricant production. Biomass Conv. Bioref. 14, 1919–1937 (2024). https://doi.org/10.1007/s13399-022-02517-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02517-x