Abstract
In this work, the autohydrolysis of Ziziphus lotus has been used as a pretreatment that permitted the integral revalorisation of this feedstock by obtaining oligosaccharides, lignin and cellulose nanofibres. For that, firstly, the temperature of the autohydrolysis process was optimised to maximise the production of oligosaccharides, obtaining 17.8 g/L of oligosaccharides at the optimum conditions. Afterwards, the effect of the autohydrolysis pretreatment on the effectiveness of the subsequent biorefinery steps and on the characteristics of the obtained products was analysed. It was observed that the autohydrolysis pretreatment not only increased the organosolv delignification process by 42.7%, but also decreased polydispersity of the obtained lignin by 33.82%. Regarding the cellulose nanofibres, their crystallinity index increased from 74.3 to 79.2% due to the effect of the autohydrolysis treatment. Thus, the autohydrolysis pretreatment would permit the integral revalorisation of Ziziphus lotus by a biorefinery approach that could be an example of the circular economy and of a zero-waste production.
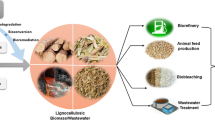
Graphical abstract








Similar content being viewed by others
Data availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
References
Zorpas AA, Lasaridi K, Abeliotis K, Voukkali I, Loizia P, Fitiri L, Bikaki N (2014) Waste prevention campaign regarding waste framework directive. Fresenius Environ Bull 23:2876–2883
Maraghni M, Gorai M, Neffati M (2010) Seed germination at different temperatures and water stress levels, and seedling emergence from different depths of Ziziphus lotus. S Afr J Bot 76:453–459. https://doi.org/10.1016/j.sajb.2010.02.092
Richardson JE, Chatrou LW, Mols JB, Erkens RHJ, Pirie MD (2004) Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. Philos Trans R Soc B 359:1495–1508. https://doi.org/10.1098/rstb.2004.1537
Hammi KM, Jdey A, Abdelly C, Majdoub H, Ksouri R (2015) Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Ziziphus lotus fruits using response surface methodology. Food Chem 184:80–89. https://doi.org/10.1016/j.foodchem.2015.03.047
Ghazghazi H, Aouadi C, Riahi I, Maaroufi A, Hasnaoui B (2014) Fatty acids composition of Tunisian Ziziphus lotus L, (Desf.) fruits and variation in biological activities between leaf and fruit extracts. Nat Prod Res 28:1106–1110. https://doi.org/10.1080/14786419.2014.913244
El Hachimi F, Alfaiz C, Bendriss A, Cherrah Y, Alaoui K (2016) Anti-inflammatory activity of the seed oil of Ziziphus lotus (L.) Desf. Pharmacognosie 15:147–154. https://doi.org/10.1007/s10298-016-1056-1
Hammi KM, Hammami M, Rihouey C, Le Cerf D, Ksouri R, Majdoub H (2018) GC-EI-MS identification data of neutral sugars of polysaccharides extracted from Ziziphus lotus Fruit. Data Brief 18:680–683. https://doi.org/10.1016/j.dib.2018.01.085
Renault HJ, Ghedira K, Thepenier P, Lavaud C, Zeches-Harnot M, Men-olivier L (1997) Dammarane sponins from Ziziphus lotus. Plant Chem 44:1321–1327. https://doi.org/10.1016/s0031-9422(96)00721-2
Rodríguez-Seoane P, Díaz-Reinoso B, Moure A, Domínguez H (2020) Potential of Paulownia sp. For biorefinery. Ind Crops Prod 155:112739. https://doi.org/10.1016/j.indcrop.2020.112739
Gullón P, Gullón B, Dávila I, Labidi J, Gonzalez-García S (2018) Comparative environmental Life Cycle Assessment of integral revalorization of vine shoots from a biorefinery perspective. Sci Total Environ 624:225–240. https://doi.org/10.1016/j.scitotenv.2017.12.036
Naidu DS, Hlangothi SP, John MJ (2018) Bio-based products from xylan: a review. Carbohydr Polym 179:28–41. https://doi.org/10.1016/j.carbpol.2017.09.064
Dávila I, Gordobil O, Labidi J, Gullón P (2016) Assessment of suitability of vine shoots for hemicellulosic oligosaccharides production through aqueous processing. Bioresour Technol 211:636–644. https://doi.org/10.1016/j.biortech.2016.03.153
Cocero MJ, Cabeza Á, Abad N, Adamovic T, Vaquerizo L, Martínez CM, Pazo-Ceped V (2018) Understanding biomass fractionation in subcritical & supercritical water. J Supercrit Fluid 133:550–565. https://doi.org/10.1016/j.supflu.2017.08.012
Chemin M, Wirotius AL, Ham-Pichavant F, Chollet G, Da Silva D, Petit-Conil M, Cramail H, Grelier S (2015) Well-defined oligosaccharides by mild acidic hydrolysis of hemicelluloses. Eur Polym J 66:190–197. https://doi.org/10.1016/j.eurpolymj.2015.02.008
Gomes ED, Rodrigues AE (2020) Recovery of vanillin from kraft lignin depolymerization with water as desorption eluent. Sep Purif Technol 239: 116551. https://doi.org/10.1016/j.seppur.2020.116551
Wang H, Xie H, Du H, Wang X, Liu W, Duan Y, Zhang X, Sun L, Zhang X, Si C (2020) Highly efficient preparation of functional and thermostable cellulose nanocrystals via H2SO4 intensified acetic acid hydrolysis. Carbohydr Polym 8:16691–16700. https://doi.org/10.1016/j.carbpol.2020.116233
Dávila I, Gullón P, Andrés MA, Labidi J (2017) Coproduction of lignin and glucose from vine shoots by eco-friendly strategies: toward the development of an integrated biorefinery. Bioresour Technol 244:328–337. https://doi.org/10.1016/j.biortech.2017.07.104
Gullόn B, Yáñez R, Alonso JL, Párajό JC (2010) Production of oligosaccharides and sugars from rye straw: a kinetic approach. Bioresour Technol 101:6676–6684. https://doi.org/10.1016/j.biortech.2010.03.080
Pan X, Gilkes N, Kadla J, Pye K, Saka S, Gregg D, Ehara K, Xie D, Lam D, Saddler J (2006) Biocoversion of hybrid poplar to ethanol and co-products using an organosolv fractionation process: optimization of process yields. Biotechnol Bioeng 94:852–861. https://doi.org/10.1002/bit.20905
Erdocia X, Prado R, Corcuera MÁ, Labidi J (2014) Effect of different organosolv treatments on the structure and properties of olive tree pruning lignin. Ind Eng Chem 20:1103–1108. https://doi.org/10.1016/j.jiec.2013.06.048
Dávila I, Remón J, Gullón P, Labidi J, Budarin V (2019) Production and characterization of lignin and cellulose fractions obtained from pretreated vine shoots by microwave assisted alkali treatment. Bioresour Technol 289:121726. https://doi.org/10.1016/j.biortech.2019.121726
Del Río JC, Gutierrez A, Hernando M, Landín P, Romero AT, Martínez ÁT (2005) Determining the influence of eucalypt lignin composition in paper pulp yield using Py-GC/MS. J Anal Appl Pyrolysis 74:110–115. https://doi.org/10.1016/j.jaap.2004.10.010
Fernández-Rodríguez J, Gordobil O, Robles E, González Alriols M, Labidi J (2017) Lignin valorization from side-streams produced during agricultural waste pulping and total chlorine free bleaching. J Clean Prod 142:2609–2617. https://doi.org/10.1016/j.jclepro.2016.10.198
Chen L, Wang X, Yang H, Lu Q, Li D, Yang Q, Chen H (2015) Study on pyrolysisbehaviors of non-woody lignins with TG-FTIR and PY-GC/MS. J Anal Appl Pyrolysis 113:499–507. https://doi.org/10.1016/j.jaap.2015.03.018
Wise L, Murphy E, Addieco MAA (1946) Clorite holocellulose: its fractionation and bearing on summative wood analysis and on studies on the hemicellulose. Pap Trade J 122:34–43
Bettaib F, Khiari R, Hassan LM, Belgacem N (2015) Preparation and characterization of new cellulose nanocrystals from marine biomass Posidonia oceanica. Ind Crops Prod 72:175–182. https://doi.org/10.1016/j.indcrop.2014.12.038
Karimi K, Taherzadeh MJ (2016) A critical review of analytical methods in pretreatment of lignocelulloses: composition, imaging and crystallinity. Bioresour Technol 200:1008–1018. https://doi.org/10.1016/j.biortech.2015.11.022
Moussaoui Y, Ferhi F, Elaloui E, Ben Salem R, Belgacem MN (2011) Utilisation of Astragalus armatus roots in papermaking. BioResources 6:4969–4978
Ferhi F, Das S, Elaloui E, Moussaoui Y, Yanez JG (2014) Chemical characterisation and suitability for papermaking applications studied on four species naturally growing in Tunisia. Ind Crops Prod 61:180–185. https://doi.org/10.1016/j.indcrop.2014.07.001
Mannai F, Ammar M, Yanez JG, Elaloui E, Moussaoui Y (2016) Cellulose fiber from Tunisian Barbary Fig"Opuntia Ficus-indica" for papermaking. Cellulose 23:2061–2072. https://doi.org/10.1007/s10570-016-0899-9
Sixta H (2006) Introduction. In: Sixta H (ed) Handbook of Pulp. WILEY-VCH, Weinheim, pp 2–19
Svärd A, Brannvall E, Edlund U (2015) Rapeseed straw as a renewable source of hemicelluloses: extraction, characterization and film formation. Carbohydr Polym 133:179–186. https://doi.org/10.1016/j.carbpol.2015.07.023
Rico X, Gullόn B, Alonso JL, Parajό JC, Yánez R (2018) Valorization of peanut shells: manufacture of bioactive oligosaccharides. Carbohydr Polym 183:21–28. https://doi.org/10.1016/j.carbpol.2017.11.009
Morales A, Hernández-Ramos F, Sillero L, Fernández-Marín R, Dávila I, Gullόn P, Erdocia X, Labidi J (2020) Multiproduct biorefinery based on almond shells: impact of the delignification stage on the manufacture of valuable products. Bioresour Technol 315:123896. https://doi.org/10.1016/j.biortech.2020.123896
Belouadah Z, Atia A, Rokbi M (2015) Characterization of new natural cellulosic fiber from Lygeum Spartum L. Carbohydr Polym 134:429–437. https://doi.org/10.1016/j.carbpol.2015.08.024
Gan T, Zhou Q, Su C, Xia J, Xie D, Liu Z, Cao Y (2021) Efficient isolation of organosolv lignin-carbohydrate complexes (LCC) with high antioxidative activity via introducing LiCl/DMSO dissolving. Int J BiolMacromol 181:752–761. https://doi.org/10.1016/j.ijbiomac.2021.03.167
Lu Y, Joosten L, Donker J, Andriulo F, Slaghek TM, Philips-Jones MK, Gosselink RJA, Harding SE (2020) Characterisation of mass distributions of solvent-fractionated lignins using analytical ultracentrifugation and size exclusion chromatography methods. Sci Rep 1:13937. https://doi.org/10.1038/s41598-021-93424-0
Ma Z, Sun Q, Ye J, Yao Q, Zhao C (2016) Study on the thermal degradation behaviours and kinetics of alkali lignin for production of phenolic-rich bio-oil using TGA-FTIR and Py-GC/MS. J Anal Appl Pyrolysis 117:116–124. https://doi.org/10.1016/j.jaap.2015.12.007
Varshney S, Mishra N, Gupta MK (2021) Progress in nanocellulose and its polymer based composites: a review on processing, characterization, and applications. Polym Comp 42:3660–3686. https://doi.org/10.1002/pc.260903660
Abu-Thabit NY, Judeh AA, Hakeel AS, Ul-Hamid A, Umar Y, Ahmed A (2020) Isolation and characterization of microcrystalline cellulose from date seeds (Phoenix dactylifera L.). Int J Biol Sci 155:730–739. https://doi.org/10.1016/j.ijbiomac.2020.03.255
Khalil HPSA, Davoudpour Y, Islam MN, Mustapha A, Sudesh K, Dungani R, Jawaid M (2014) Production and modification of nanofibrillated cellulose using various mechanical processes: a review. Carbohydr Polym 99:649–665. https://doi.org/10.1016/j.carbpol.2013.08.069
Guancha-Chalapud MA, Gálvez J, Serna-Cock L, Aguilar CN (2020) Valorization of Colombian fique (Furcraea bedinghausii) for production of cellulose nanofibers and its application in hydrogels. Sci Rep 10:11637. https://doi.org/10.1038/s41598-020-68368-6
Ejeta KO, Azeez TO, Banigo AT, Nkuma-Udah KI, Ajuogu E (2020) Isolation and characterization of cellulose nanofibres from three common Nigerian grasses. Mater Sci Eng, IOP Conf Series. https://doi.org/10.1088/1757-899X/805/1/012040
Nie S, Zhang K, Lin X, Zhang C, Yan D, Liang H, Wang S (2018) Enzymatic pretreatment for the improvement of dispersion and film properties of cellulose nanofibrils. Carbohydr Polym 181:1136–1142. https://doi.org/10.1016/j.carbpol.2017.11.020
Syafri E, Kasim A, Abral H, Asben A (2019) Cellulose nanofibers isolation and characterization from ramie using a chemical-ultrasonic treatment. J Nat Fibers 16:1145–1155. https://doi.org/10.1080/15440478.2018.1455073
Xiao Y, Liu Y, Wang X, Li H (2019) Cellulose nanocrystals prepared from wheat bran: characterization and cytotoxicity assessment. Int J Biol Macromol 140:225–233. https://doi.org/10.1016/j.ijbiomac.2019.08.160
Kamelina E, Divsalar A, Darroudi M, Yaghmaei P, Sadri K (2019) Production of new cellulose nanocrystals from ferula gummosa and their use in medical applications via investigation of their biodistribution. Ind Crops Prod 139: 111538. https://doi.org/10.1016/j.indcrop.2019.111538
Acknowledgements
The authors greatly acknowledge the financial support of the Ministry of Higher Education and Scientific Research of Tunisia. Dr. I. Dávila would like to thank the University of the Basque Country (UPV/EHU) for the financial support (Grant reference DOCREC19/47). The authors also want to acknowledge SGIker services from the University of the Basque Country UPV/EHU for their help with AFM, XRD and TGA analyses.
Funding
This work was supported by the Ministry of Higher Education and Scientific Research of Tunisia and the Department of Chemical and Environmental Engineering, Biorefinery Processes Research Group, University of the Basque Country, Spain.
Author information
Authors and Affiliations
Contributions
Ideation and design of the experiment were done by Sara Saad and Izaskun Dávila. Development and optimization of experimental methods were done by Sara Saad and Izaskun Dávila. Collection and interpretation of experimental data was done by Sara Saad, Izaskun Dávila, Faten Mannai and Jalel Labidi. Preparing and writing of the manuscript were done by Sara Saad, Izaskun Dávila, Younes Moussaoui and Jalel Labidi.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. Hence, no formal consent is required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Ziziphus lotus was converted into oligosaccharides, lignin and cellulose nanofibres.
• The autohydrolysis process increased the efficiency of the subsequent delignification by 42.7%.
• Nanofibres with higher crystallinity (79.2% vs 74.3%) were achieved after the hemicellulose removal.
Rights and permissions
About this article
Cite this article
Saad, S., Dávila, I., Mannai, F. et al. Effect of the autohydrolysis treatment on the integral revalorisation of Ziziphus lotus. Biomass Conv. Bioref. 14, 1413–1425 (2024). https://doi.org/10.1007/s13399-022-02457-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02457-6