Abstract
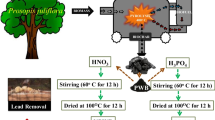
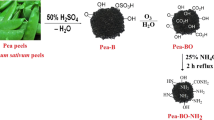
While various techniques have been explored for the sequestration of heavy metals such as copper from industrial effluent, there is enormous research on the use of biosorbents created from bio-waste in the environment for the confiscation of Cu(II) ion owing to their low cost and green nature. In this study, a biochar-NH2 biosorbent synthesized from watermelon peels was explored for the elimination of Cu(II) ion from water-soluble solutions. The synthesized biochar-NH2 was further characterized using FTIR, BET, and SEM coupled with EDX to ascertain the present functional groups, the surface area, morphology, and elementary composition of the biosorbent. The determined biochar-NH2 BET-specific surface area and the total pore volume were 12.26 m2/g and 0.012 cm3/g. The differential thermal analysis (DTA) of the WWP and biochar-NH2 samples showed six peaks at flow temperatures of 769, 178, 251, 292, 426, and 872 °C, and two distinct degradation peaks at flow temperatures of 767 and 477 °C. The sequestration of Cu(II) was noted in this study to be dependent on the pH, with the best possible confiscation of Cu(II) ion noticed at pH 5.6. The process of Cu(II) adsorption to the biosorbent was perfectly defined utilizing the Langmuir (LGR) and pseudo-second-order (PSO) models with correlation coefficient (R2) values of 0.944 and < 0.99. It is implicit that the sorption process consists of a chemisorption process in rate-limiting stages. The determined sorption capacity estimated from the Langmuir isotherm model was determined to be 140.85 mg/g and when the sorption capacity of biochar-NH2 was compared to other biosorbents employed for the sequestration of Cu(II) ion from water-soluble solutions, the biochar-NH2 biosorbent was found to be an effective biosorbent for Cu(II) elimination. Additionally, it was found that the biosorbent can be regenerated up to six regeneration cycles for the confiscation of Cu(II) ion. In conclusion, it was discovered in this study that the synthesized biosorbent was a cost-effective adsorbent with a high adsorption efficacy for Cu(II) removal.










Similar content being viewed by others
Data availability
Data sharing does not apply to this article.
References
Ukhurebor KE, Athar H, Adetunji CO, Aigbe UO, Onyancha RB, Abifarin O (2021) Environmental implications of petroleum spillages in the Niger Delta region of Nigeria: a review. J Environ Managem 293:112872
Onyancha RB, Aigbe UO, Ukhurebor KE, Muchiri PW (2021) Facile synthesis and applications of carbon nanotubes in heavy metal remediation and biomedical fields: a comprehensive review. J Mol Struct 1238:130462
Onyancha RB, Ukhurebor KE, Aigbe UO, Osibote OA, Kusuma HS, Darmokoesoemo H, Balogun VA (2021) A systematic review on the detection and monitoring of toxic gases using carbon nanotube-based biosensors. Sens Bio-Sens Res 34:100463
Ukhurebor KE, Aigbe UO, Onyancha RB, Nwankwo W, Osibote OA, Paumo HK, Ama OM, Adetunji CO, Siloko IU (2021) Effect of hexavalent chromium on the environment and removal techniques: a review. J Environ Managem 280:111809
Aigbe UO, Onyancha RB, Ukhurebor KE, Obodo KO (2020) Removal of fluoride ions using polypyrrole magnetic nanocomposite influenced by rotating magnetic field. RSC Adv 10(1):595–609
El Nemr A (2011) Impact, monitoring and management of environmental pollution, Nova Science Publishers, Inc. Hauppauge New York. [ISBN-10: 1608764877, ISBN-13: 9781608764877] 1–638
El Nemr A (2012) Environmental pollution and its relation to climate change, Nova Science Publishers, Inc. Hauppauge New York. [ISBN-13: 978–1–61761–794–2] 694 pages
Rezania S, Taib SM, Din MFM, Dahalan FA, Kamyab H (2016) Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J Hazard Mater 318:587–599
Sudarni DHA, Aigbe UO, Ukhurebor KE, Onyancha RB, Kusuma HS, Darmokoesoemo H, Osibote OA, Balogun VA, Widyaningrum BA (2021) Malachite green removal by activated potassium hydroxide clove leaves agro-waste biosorbent: characterization, kinetics, isotherms and thermodynamics studies. Adsorp Sci Technol 1145312:1–15
Ben-Ali S, Jaouali I, Souissi-Najar S, Ouederni A (2017) Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J Cleaner Product 142:3809–3821
Kerry RG, Ukhurebor KE, Kumari S, Maurya GK, Patra S, Panigrahi B, Majhi S, Rout JR, Rodriguez-Torres MDP, Das G, Shin H-S, Patra JK (2021) A comprehensive review on the applications of nano-biosensor based approaches for non-communicable and communicable disease detection. Biomater Sci 9:3576–3602
Ukhurebor KE, Singh KRB, Nayak V, UK-Eghonghon G, (2021) Influence of SARS-CoV-2 pandemic: a review from the climate change perspective. Environ Sci: Proces Impacts 23:1060–1078
Ukhurebor KE, Azi SO, Aigbe UO, Onyancha RB, Emegha JO (2020) Analysing the uncertainties between reanalysis meteorological data and ground measured meteorological data. Measurement 165:108110
Phuengphai P, Singjanusong T, Kheangkhun N, Amnuay Wattanakornsiri A (2021) Removal of copper (II) from aqueous solution using chemically modified fruit peels as efficient low-cost biosorbents. Water Sci Eng. https://doi.org/10.1016/j.wse.2021.08.003
Ukhurebor KE, Aidonojie PA (2021) The influence of climate change on food innovation technology: review on topical developments and legal framework. Agric & Food Secur 10(50):1–14. https://doi.org/10.1186/s40066-021-00327-4
Gupta H, Parag R, Gogate PR (2016) Intensified removal of copper from waste water using activated watermelon based biosorbent in the presence of ultrasound. Ultrasonics Sonochem 30:113–122
Khaled A, El Nemr A, El Sikaily A (2013) Heavy metals concentrations in biota of the Mediterranean Sea: a review. Part I Blue Biotech J 2(1):79–133
Khaled A, El Nemr A, El Sikaily A (2013) Heavy metals concentrations in biota of the Mediterranean Sea: a review, Part II. Blue Biotech J 2(2):191–249
Doebrich J, Linda Masonic L (2009) Copper—a metal for the ages. United States Geological Survey, Fact Sheet 2009–3031, May 2009. https://pubs.usgs.gov/fs/2009/3031/FS2009-3031. Accessed 16 Jan 2022
El-Sikaily A, El Nemr A, Khaled A (2011) Copper sorption onto dried red alga Pterocladia capillacea and its activated carbon. Chem Eng J 168:707–714
El Nemr A, El Sadaawy MM, Khaled A, El Sikaily A (2014) Adsorption of the anionic dye Direct Red 23 onto new activated carbons developed from Cynara cardunculus: kinetics, equilibrium and thermodynamics. Blue Biotech J 3(1):121–142
Villen-Guzman M, Gutierrez-Pinilla D, Gomez-Lahoz C, Vereda-Alonso C, Rodriguez-Maroto JM, Arhoun B (2019) Optimization of Ni(II) biosorption from aqueous solution on modified lemon peel. Environ Res 179:108849
Ngabura M, Hussain SA, Ghani WAWA, Jami MS, Tan YP (2018) Utilization of renewable durian peels for biosorption of zinc from wastewater. J Environ Chem Eng 6(2):2528–2539
Alghamdi MM, El-Zahhar AA, Idris AM, Sadi TO, Sahlabji T, El Nemr A (2019) Synthesis, characterization and application of a new polymeric-clay-magnetite composite resin for water softening. Sep Purif Technol 224:356–365. https://doi.org/10.1016/j.seppur.2019.05.037
Chen Q, Yao Y, Li X, Lu J, Zhou J, Huang Z (2018) Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J Water Process Eng 26:289–300
Wu H, Wang W, Huang Y, Han G, Yang S, Su S, Sana H, Peng W, Cao Y, Liu J (2019) Comprehensive evaluation on a prospective precipitation-flotation process for metal-ions removal from wastewater simulants. J Hazard Mater 371:592–602
Yesil H, Tugtas AE (2019) Removal of heavy metals from leaching effluents of sewage sludge via supported liquid membranes. Sci Total Environ 693:133608
Guyo U, Mhonyera J, Moyo M (2015) Pb(II) adsorption from aqueous solutions by raw and treated biomass of maize stover: a comparative study. Process Safety Environ Protect 93:192–200
Khaled A, El Nemr A, El-Sikaily A, Abdelwahab O (2005) Biosorption of basic blue nine from water solution by marine algae. Egypt J Aquat Res 31:130–141
Aksu Z, İşoğlu İA (2005) Removal of copper (II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochem 40(9):3031–3044
El Nemr A, Abdelwahab AO, Khaled A, El-Sikaily A (2005) Removal of chrysophenine from aqueous solution by Ulva Lactuca. Egypt J Aquat Res 31:86–98
Keawkim K, Khamthip A (2018) Removal of Pb2+ ion from industrial wastewater by new efficient biosorbents of Oyster plant (Tradescantia spathacea Steam) and Negkassar leaf (Mammea siamensis T. Anderson). Chiang Mai J Sci 45(1):369–379
Sun H, Xia N, Liu Z, Kong F, Wang S (2019) Removal of copper and cadmium ions from alkaline solutions using chitosan-tannin functional paper materials as adsorbent. Chemosphere 236:124370
Ismael MN, El Nemr A, El Sayed H, Hamid HA (2020) Removal of hexavalent chromium by cross-linking chitosan and N, N’-methylene bis-acrylamide. Environ Process 7:911–930. https://doi.org/10.1007/s40710-020-00447-2
Hosain AN, El Nemr A, El Sikaily A, Mahmoud ME, Amira MF (2020) Surface modifications of nanochitosan coated magnetic nanoparticles and their applications in Pb(II), Cu(II) and Cd(II) removal. J Environ Chem Eng 8(5):104316. https://doi.org/10.1016/j.jece.2020.104316
El Nemr A, El-Assal AA, El Sikaily A, Mahmoud ME, Amira MF, Ragab S (2021) New magnetic cellulose nanobiocomposites for Cu(II), Cd(II) and Pb(II) ions removal: kinetics, thermodynamics and analytical evaluation. Nanotech Environ Eng 6(3):1–20. https://doi.org/10.1007/s41204-021-00138-9
Manzoor Q, Nadeem R, Iqbal M, Saeed R, Ansari TM (2013) Organic acids pretreatment effect on Rosa bourbonia phyto-biomass for removal of Pb(II) and Cu (II) from aqueous media. Bioresour Technol 132:446–452
Tasaso P (2014) Adsorption of copper using pomelo peel and depectinated pomelo peel. J Clean Energy Tech 2(2):154–157
Romero-Cano LA, García-Rosero H, Gonzalez-Gutierrez LV, Baldenegro-Pérez LA, Carrasco-Marín F (2017) Functionalized adsorbents prepared from fruit peels: equilibrium, kinetic and thermodynamic studies for copper adsorption in aqueous solution. J Cleaner Prod 162:195–204
Safari E, Rahemi N, Kahforoushan D, Allahyari S (2019) Copper adsorptive removal from aqueous solution by orange peel residue carbon nanoparticles synthesized by combustion method using response surface methodology. J Environ Chem Eng 7(1):102847
Semerciöz AS, Göğüş F, Çelekli A, Bozkurt H (2017) Development of carbonaceous material from grapefruit peel with microwave implemented-low temperature hydrothermal carbonization technique for the adsorption of Cu(II). J Cleaner Prod 165:599–610
Zhang W, Song J, He Q, Wang H, Lyu W, Feng H, Xiong W, Guo W, Wu J, Chen L (2020) Novel pectin based composite hydrogel derived from grapefruit peel for enhanced Cu(II) removal. J Hazard Mater 384:121445
El-Nemr MA, Abdelmonem NM, Ismail IM, Ragab S, El Nemr A (2020) Ozone and ammonium hydroxide modification of biochar prepared from Pisum sativum peels improves the adsorption of copper (II) from an aqueous medium. Environ Process 7(3):973–1007. https://doi.org/10.1007/s40710-020-00455-2
Eldeeb TM, El Nemr A, Khedr MH, El-Dek SI (2021) Efficient removal of Cu(II) from water solution using magnetic chitosan nanocomposite. Nanotechnol Environ Eng 6(2):1–15. https://doi.org/10.1007/s41204-021-00129-w
Eldeeb TM, El-Nemr A, Khedr MH, El-Dek SI (2021) Novel bio-nanocomposite for efficient copper removal. Egypt J Aquat Res 47(3):261–267. https://doi.org/10.1016/j.ejar.2021.07.002
Gregg SJ, Sing KSW (1982) Adsorption surface area and porosity, 2nd edn. Academic Press INC., London, pp 957–957
Rouquerol F, Rouquerol J, Sing KSW (1999) Adsorption by powders and porous solids. Academic Press INC., London
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Amer Chem Soc 73(1):373–380
El-Nemr MA, Abdelmonem NM, Ismail IMA, Ragab S, El Nemr A (2020) Removal of acid yellow 11 dye using novel modified biochar derived from watermelon peels. Desal Water Treat 203:403–431
El-Nemr MA, Ismail IMA, Abdelmonem NM, El Nemr A, Ragab S (2021) Amination of biochar derived from watermelon peel by triethylenetetramine and ammonium hydroxide for toxic chromium removal enhancement. Chin J Chem Eng 36:199–222
Duan C, Ma T, Wang J, Zhou Y (2020) Removal of heavy metals from aqueous solution using carbon-based adsorbents: a review. J Water Process Eng 37:101339
El Nemr A, Shoaib AGM, El Sikaily A, Ragab S, Mohamed AE-DA, Hassan AF (2021) Utilization of green alga Ulva lactuca for sustainable production of meso-micro porous nano activated carbon for adsorption of direct red 23 dye from aquatic environment. Carb Lett. https://doi.org/10.1007/s42823-021-00262-1 (In press)
Carvalho JTT, Milani PA, Consonni JL, Labuto G, Carrilho ENVM (2021) Nanomodified sugarcane bagasse biosorbent: synthesis, characterization, and application for Cu (II) removal from aqueous medium. Environ Sci Poll Res 28(19):24744–24755
Shoaib AG, El-Sikaily A, El Nemr A, Mohamed AEDA, Hassan AA (2020) Preparation and characterization of highly surface area activated carbons followed type IV from marine red alga (Pterocladia capillacea) by zinc chloride activation. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00760-8 (in press)
Shoaib AG, El-Sikaily A, El Nemr A, Mohamed AEDA, Hassan AA (2020) Testing the carbonization condition for high surface area preparation of activated carbon followed type IV from green alga Ulva Lactuca. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00823-w (in press)
Aigbe UO, Osibote OA (2021) Carbon derived nanomaterials for the sorption of heavy metals from aqueous solution: a review. Environ Nanotechnol Monitor Manag 16:100578
Arslanoğlu H, Kaya S, Tümen F (2020) Cr (VI) adsorption on low-cost activated carbon developed from grape marc-vinasse mixture. Particul Sci Technol 38(6):768–781
Aigbe UO, Osibote OA (2020) A review of hexavalent chromium removal from aqueous solutions by sorption technique using nanomaterials. J Environ Chem Eng 8:104503
Eleryan A, El Nemr A, Idris AM, Alghamdi MM, El-Zahhar AA, Said TO, Sahlabji T (2020) Feasible and eco-friendly removal of hexavalent chromium toxicant from aqueous solutions using chemically modified sugarcane bagasse cellulose. Toxin Rev. https://doi.org/10.1080/15569543.2020.1790606 (in press)
El Nemr A, Aboughaly RM, El Sikaily A, Ragab S, Masoud MS, Ramadan MS (2020) Microporous nano activated carbon type I derived from orange peel and its application for Cr(VI) removal from aquatic environment. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00995-5 (in press)
El Nemr A, Aboughaly RM, El Sikaily A, Ragab S, Masoud MS, Ramadan MS (2021) Utilization of sugarcane bagasse/ZnCl2 for sustainable production of microporous nano activated carbons of type I for toxic Cr(VI) removal from aqueous environment. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01445-6
Mohamad OA, Hao X, Xie P, Hatab S, Lin Y, Wei G (2009) Biosorption of copper (II) from aqueous solution using non-living mesorhizobium amorphae strain CCNWGS0123. Microbes Environ 27(3):234–241. https://doi.org/10.1264/jsme2.ME11331
Sahlabji T, El-Nemr MA, El Nemr A, Ragab S, Alghamdi MM, El-Zahhar AA, Idris AM, Said TO (2021) High surface area microporous activated carbon from Pisum sativum peels for hexavalent chromium removal from aquatic environment. Toxin Rev. https://doi.org/10.1080/15569543.2021.1908361 (in press)
Wang G, Zhang S, Yao P, Chen Y, Xu X, Li T, Gong G (2018) Removal of Pb (II) from aqueous solutions by Phytolacca americana L. biomass as a low cost biosorbent. Arab J Chem 11(1):99–110
Ismail MNM, El Nemr A, El Ashry ESH, Abdel Hamid H (2020) Novel simple modification of chitosan as adsorptive agent for removal of Cr6+ from aqueous solution. Egypt J Chem 63(4):1219–1240. https://doi.org/10.21608/ejchem.2019.11157.1716
Duan C, Ma T, Wang J, Zhou Y (2020) Removal of heavy metals from aqueous solution using carbon-based adsorbents: a review. J Water Process Eng 37:101339
Mohammed TJ, Ibrahim RI (2016) Remediation of Cu (II) from well water of Iraq by using cortex of fruits and agricultural waste. Arab J Sci Eng 41(2):345–355
Foroutan R, Peighambardoust SJ, Peighambardoust SH, Pateiro M, Lorenzo JM (2021) Adsorption of crystal violet dye using activated carbon of lemon wood and activated carbon/Fe3O4 magnetic nanocomposite from aqueous solutions: a kinetic, equilibrium and thermodynamic study. Molecules 26(8):2241
Benzaoui T, Selatnia A, Djabali D (2018) Adsorption of copper (II) ions from aqueous solution using bottom ash of expired drugs incineration. Adsorpt Sci Technol 36(1–2):114–129
El Nemr A, El-Sikaily A, Khaled A (2010) Modeling of adsorption isotherms of methylene blue onto rice husk activated carbon. Egypt J Aquat Res 36(3):403–425
Banerjee K, Ramesh ST, Gandhimathi R, Nidheesh PV, Bharathi KS (2012) A novel agricultural waste adsorbent, watermelon shell for the removal of copper from aqueous solutions. Iranica J Ener Environ 3(2):143–156
Hassaan MA, El Nemr A, Madkour FF (2017) Testing the advanced oxidation processes on the degradation of direct blue 86 dye in wastewater. Egypt J Aquat Res 43:11–19. https://doi.org/10.1016/j.ejar.2016.09.006
Hassaan MA, El Nemr A, Madkour FF (2017) Advanced oxidation processes of mordant violet 40 dye in freshwater and seawater. Egypt J Aquat Res 43(1):1–9. https://doi.org/10.1016/j.ejar.2016.09.004
Grover A, Mohiuddin I, Malik AK, Aulakh JS, Vikrant K, Kim KH, Brown RJ (2021) Magnesium/aluminum layered double hydroxides intercalated with starch for effective adsorptive removal of anionic dyes. J Hazard Mater 424:127454
Adebayo GB, Jamiu W, Okoro HK, Okeola FO, Adesina AK, Feyisetan OA (2019) Kinetics, thermodynamics and isothermal modelling of liquid phase adsorption of methylene blue onto moringa pod husk activated carbon. South Afr J Chem 72:263–237
Fernandes T, Soares SF, Trindade T, Daniel-da-Silva AL (2017) Magnetic hybrid nanosorbents for the uptake of paraquat from water. Nanomaterials 7(3):68
Jadhav AS, Mohanraj GT (2017) Evaluation of kinetic models of lead uptake from wastewater by activated carbon derived from coconut leaves. Nature Environ Pollut Technol 6(1):189–197
López-Luna J, Ramírez-Montes LE, Martinez-Vargas S, Martínez AI, Mijangos-Ricardez OF, María del Carmen A, Carrillo-González R, Solís-Domínguez FA, del Carmen C-Díaz M, Vázquez-Hipólito V (2019) Linear and nonlinear kinetic and isotherm adsorption models for arsenic removal by manganese ferrite nanoparticles. SN Appl Sci 1(8):1–19
Ofomaja AE, Naidoo EB, Pholosi A (2020) Intraparticle diffusion of Cr (VI) through biomass and magnetite coated biomass: a comparative kinetic and diffusion study. South Afr J Chem Eng 32(1):39–55
Cao Q, Huang Z, Liu S, Wu Y (2019) Potential of Punica granatum biochar to adsorb Cu (II) in soil. Sci Rep 9(1):1–13
Taib NI, Rosli NA, Saharrudin NI, Rozi NM, Kasdiehram NAA, Abu Nazri NNT (2021) Kinetic, equilibrium, and thermodynamic studies of untreated watermelon peels for removal of copper (II) from aqueous solution. Desal Water Treat 227:289–299
Lucaci AR, Bulgariu D, Ahmad I, Bulgariu L (2020) Equilibrium and kinetics studies of metal ions biosorption on alginate extracted from marine red algae biomass (Callithamnion corymbosum sp.). Polymers 12(9):1888
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and interpretation of adsorption isotherms. J Chem 2017:3039817. https://doi.org/10.1155/2017/3039817
Hossain MA, Ngo HH, Guo WS, Nguyen TV (2012) Biosorption of Cu (II) from water by banana peel based biosorbent: experiments and models of adsorption and desorption. J Water Sustain 2(1):87–104
Bin Yu YZ, Shukla A, Shukla SS, Dorris KL (2001) The removal of heavy metals from aqueous solutions by sawdust adsorption—removal of lead and comparison of its adsorption with copper. J Hazard Mater B 84:83–94
Shahtalebi A, Sarrafzadeh MH, McKay G (2013) An adsorption diffusion model for removal of copper (II) from aqueous solution by pyrolytic tyre char. Desal Water Treat 51(28–30):5664–5673
Ibrahim RI (2020) Optimization process for removing of copper ions from groundwater of Iraq using watermelon shells as natural adsorbent. IOP Conf Ser Mater Sci Eng 737(1):012195
Lakshmipathy R, Sarada NC (2016) Metal ion free watermelon (Citrullus lanatus) rind as adsorbent for the removal of lead and copper ions from aqueous solution. Desalin Water Treat 57(33):15362–15372
Gupta H, Gogate PR (2016) Intensified removal of copper from waste water using activated watermelon based biosorbent in the presence of ultrasound. Ultrason Sonochem 30:113–122
Banerjee K, Ramesh ST, Gandhimathi R, Nidheesh PV, Bharathi KS (2012) A novel agricultural waste adsorbent, watermelon shell for the removal of copper from aqueous solutions. Iran J Energy Environ 3(2):143–156
Medhi H, Chowdhury PR, Baruah PD, Bhattacharyya KG (2020) Kinetics of aqueous Cu (II) biosorption onto Thevetia peruviana leaf powder. ACS Omega 5(23):13489–13502
Funding
This work was partially funded by the Science and Technology Development Fund (STDF) of Egypt (Projects Nos. CB-4874 and CB-22816).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Nemr, M.A., Aigbe, U.O., Hassaan, M.A. et al. The use of biochar-NH2 produced from watermelon peels as a natural adsorbent for the removal of Cu(II) ion from water. Biomass Conv. Bioref. 14, 1975–1991 (2024). https://doi.org/10.1007/s13399-022-02327-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02327-1