Abstract
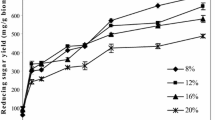
Alcoholic fermentation at high solid substrate content has been developed to enhance ethanol productivity. However, the high viscosity of fermenting mash caused several problems, including ineffective hydrolysis and low fermentation productivity, in the manufacturing process. Addition of cellulase and pectinase during mash preparation can decrease the mash viscosity. In this study, Aspergillus aculeatinus mutant strain specially developed for reducing the viscosity of cassava root mash was obtained by using the sequential mutagenesis approach. The selected mutant strain, namely SF-034, produced approximately 64.2% and 62.7% higher cellulase and pectinase under shake flask cultivation. The hyphae of the SF-034 strain have more frequent branching compared to that of the wild-type, suggesting a favorable feature for enzyme secretion at a high level. Upscaling the enzyme production in a bioreactor showed the highest cellulase and pectinase activities of 583.3 U/mL and 492.3 U/mL, respectively. The mutant crude enzyme showed a superior level of viscosity reduction efficiency (98.5%) on the treatment of cassava root mash at very high-gravity (VHG) condition. The A. aculeatinus mutant strain developed in this study has a great potential for on-site production of viscosity reduction enzymes that can be exploited for efficient VHG ethanol fermentation.





Similar content being viewed by others
References
Kularathne IW, Gunathilake C, Yatipanthalawa BS, Kalpage CS, Rathneweera AC, Rajapakse S (2019) Production of green energy – ethanol dehydration using rice straw, rice husk and castor oil. Biomass Conv Bioref. https://doi.org/10.1007/s13399-019-00560-9
Vohre M, Manwar J, Manmode R, Padgilwar S, Patil S (2014) Bioethanol production: feedstock and current technologies. J Environ Chem Eng 2:573–584. https://doi.org/10.1016/j.jece.2013.10.013
Puligundla P, Smogrovicova D, Mok C, Obulam VSR (2019) A review of recent advances in high gravity ethanol fermentation. Renew Energy 133:1366–1379. https://doi.org/10.1016/j.renene.2018.06.062
Thomas KC, Hynes SH, Jones AM, Ingledew WM (1993) Production of fuel alcohol from wheat by VHG technology. Appl Biochem Biotechnol 43:211–226
Puligundla P, Smogrovicova D, Obulam VSR, Ko S (2011) Very high gravity (VHG) ethanolic brewing and fermentation: a research update. J Ind Microbiol Biotechnol 38:1133–1144. https://doi.org/10.1007/s10295-011-0999-3
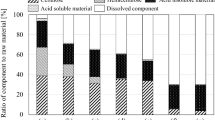
Poonsrisawat A, Wanlapatit S, Wansuksri R, Piyachomkwan K, Paemanee A, Gamonpilas C et al (2016) Synergistic effects of cell wall degrading enzymes on rheology of cassava root mash. Process Biochem 51:2104–2111. https://doi.org/10.1016/j.procbio.2016.09.010
Siriwong T, Laimeheriwa B, Aini UN, Cahyanto MN, Reungsang A, Salakkam A (2019) Cold hydrolysis of cassava pulp and its use in simultaneous saccharification and fermentation (SSF) process for ethanol fermentation. J Biotechnol 292:57–63. https://doi.org/10.1016/j.jbiotec.2019.01.003
Poonsrisawat A, Wanlapatit S, Paemanee A, Eurwilaichitr L, Piyachomkwan K, Champredaa V (2014) Viscosity reduction of cassava for very high gravity ethanol fermentation using cell wall degrading enzymes from Aspergillus aculeatus. Process Biochem 49:1950–1957. https://doi.org/10.1016/j.procbio.2014.07.016
Nguyen CN, Le TM, Chu-Ky S (2014) Pilot scale simultaneous saccharification and fermentation at very high gravity of cassava flour for ethanol production. Ind Crops Prod 56:160–165. https://doi.org/10.1016/j.indcrop.2014.02.004
Liu Y, Xu J, Zhang Y, Yuan Z, Xie J (2015) Optimization of high solids fed-batch saccharification of sugarcane bagasse based on system viscosity changes. J Biotechnol 211:5–9. https://doi.org/10.1016/j.jbiotec.2015.06.422
Poonsrisawat A, Paemanee A, Wanlapatit S, Piyachomkwan K, Eurwilaichitr L, Champreda V (2017) Simultaneous saccharification and viscosity reduction of cassava pulp using a multi-component starch- and cell-wall degrading enzyme for bioethanol production. 3 Biotech 7:290. https://doi.org/10.1007/s13205-017-0924-1
Ellilä S, Fonseca L, Uchima C, Cota J, Goldman GH, Saloheimo M et al (2017) Development of a low-cost cellulase production process using Trichoderma reesei for Brazilian biorefineries. Biotechnol Biofuels 10:30. https://doi.org/10.1186/s13068-017-0717-0
Amin F, Bhatti HN, Bilal M (2019) Recent advances in the production strategies of microbial pectinases—a review. Int J Biol Macromol 122:1017–1026. https://doi.org/10.1016/j.ijbiomac.2018.09.048
Heerd D, TariC F-L (2014) Microbial strain improvement for enhanced polygalacturonase production by Aspergillus sojae. Appl Microbiol Biotechnol 98:7471–7481. https://doi.org/10.1007/s00253-014-5657-z
Parekh S, Vinci VA, Strobel RJ (2000) Improvement of microbial strains and fermentation processes. Appl Microbiol Biotechnol 54:287–301. https://doi.org/10.1007/s002530000403
Lim J, Choi YH, Hurk BS, Lee I (2019) Strain improvement of Aspergillus sojae for increased l-leucine aminopeptidase and protease production. Food Sci Biotechnol 28:121–128. https://doi.org/10.1007/s10068-018-0427-9
Basotra N, Kaur B, Raheja Y, Agrawal D, Sharma G, Chadha BS (2021) Developing and evaluating lignocellulolytic hyper producing deregulated strains of Mycothermus thermophilus for hydrolysis of lignocellulosics. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01539-1
Syafriana V, Nuswantara S, Mangunwardoyo W, Lisdiyanti P (2014) Enhancement of B-glucosidase activity in Penicillium sp. by random mutation with ultraviolet and ethyl methyl sulfonate. Ann Bogor 18:27–33
Suri P, Dwivedi D, Rathour RK, Rana N, Sharma V, Bhatia RK et al (2021) Enhanced C-5 sugar production from pine needle waste biomass using Bacillus sp. XPB-11 mutant and its biotransformation to bioethanol. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01277-4
Kamalambigeswari R, Alagar S, Sivvaswamy N (2018) Strain improvement through mutation to enhance pectinase yield from Aspergillus niger and molecular characterization of polygalactouronase gene. J Pharm Sci Res 10:989–994
Nisar K, Abdullah R, Kaleem A, Iqtedar M, Saleem F (2020) Hyper production of carboxy methyl cellulase by Thermomyces dupontii utilizing physica and chemical mutagenesis. Rev Mex Ing Quim 19:617–62. https://doi.org/10.24275/rmiq/Bio8235
Noonim P, Mahakarnchanakul W, Varga J, Frisvad JC, Samson RA (2008) Two novel species of Aspergillus section Nigri from Thai coffee beans. Int J Syst Evol Microbiol 58:1727–1734. https://doi.org/10.1099/ijs.0.65694-0
Lemaire A, Garzon CD, Perrin A, Habrylo O, Trezel P, Bassard S et al (2020) Three novel rhamnogalacturonan I- pectins degrading enzymes from Aspergillus aculeatinus: Biochemical characterization and application potential. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2020.116752
Garzon CD, Habrylo O, Lemaire A, Guillaume A, Carre Y, Millet C et al (2021) Characterization of a novel strain of Aspergillus aculeatinus: From rhamnogalacturonan type I pectin degradation to improvement of fruit juice filtration. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2021.117943
Narra M, Dixit G, Divecha J, Madamwar D, Shah AR (2012) Production of cellulases by solid state fermentation with Aspergillus terreus and enzymatic hydrolysis of mild alkali-treated rice straw. Bioresour Technol 121:355–361. https://doi.org/10.1016/j.biortech.2012.05.140
Mandel M, Weber J (1969) The production of cellulases. Adv Chem 95:391–414. https://doi.org/10.1021/ba-1969-0095.ch023
Beguin P, Aubert JP (1994) The biological degradation of cellulose. FEMS Microbiol Lett 13:25–58. https://doi.org/10.1111/j.1574-6976.1994.tb00033.x
Mukesh Kumar DJ, Saranya GM, Suresh K, Andal Priyadharshini D, Rajakumar R, Kalaichelvan PT (2012) Production and optimization of pectinase from Bacillus sp. MFW7 using cassava waste. Asian J Plant Sci Res 2:369–375
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Schneider WDH, Goncalves TA, Uchima CA, Reis LD, Fontana RC, Squina FM et al (2018) Comparison of the production of enzymes to cell wall hydrolysis using different carbon sources by Penicillium echinulatumstrains and its hydrolysis potential for lignocelullosic biomass. Process Biochem 66:162–170. https://doi.org/10.1016/j.procbio.2017.11.004
Adsul MG, Bastawde KB, Varma AJ, Gokhale DV (2007) Strain improvement of Penicillium janthinellum NCIM 1171 for increased cellulase production. Bioresour Technol 98:1467–1473. https://doi.org/10.1016/j.biortech.2006.02.036
Aleem B, Rashid MH, Zeb N, Saqib A, Ihsan A, Iqbal M et al (2018) Random mutagenesis of super Koji (Aspergillus oryzae): improvement in production and thermal stability of α-amylases for maltose syrup production. BMC Microbiol 18:200. https://doi.org/10.1186/s12866-018-1345-y
Wosten HAB, Moukha SM, Sietsma JH, Wessels JGH (1991) Localization of growth and secretion of proteins in Aspergillus niger. J Gen Microbiol 137:2017–2023. https://doi.org/10.1099/00221287-137-8-2017
Muller C, Mclntyre M, Hansen K, Nielsen J (2002) Metabolic engineering of the morphology of Aspergillus oryzae by altering chitin synthesis. Appl Environ Microbiol 68:1827–1836. https://doi.org/10.1128/AEM.68.4.1827-1836.2002
Fiedler MRM, Barthel L, Kubisch C, Nai C, Meyer V (2018) Construction of an improved Aspergillus niger platform for enhanced glucoamylase secretion. Microb Cell Fact 17:95. https://doi.org/10.1186/s12934-018-0941-8
Ega SL, Drendel G, Petrovski S, Egidi E, Franks AE, Muddada S (2020) Comparative analysis of structural variations due to genome shuffling of Bacillus subtilis VS15 for improved cellulase production. Int J Mol Sci 21:1299. https://doi.org/10.3390/ijms21041299
Sapinska E, Balcerek M, Pielech-Przybylska K (2013) Alcoholic fermentation of high-gravity corn mashes with the addition of supportive enzymes. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.4078
Amalia AV, Fibriana F, Widiatningrum T, Hardianti RW (2021) Bioconversion and valorization of cassava-based industrial wastes to bioethanol gel and its potential application as a clean cooking fuel. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2021.102093
Acknowledgements
The authors would like to thank Dr. Piyanun Harnpicharnchai for critically reading the manuscript.
Funding
The financial support by Thailand Science Research and Innovation (TSRI) under the spearhead program (SIP6250002) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
U-thai, P., Poonsrisawat, A., Arnthong, J. et al. Enhanced viscosity reduction efficacy of cassava root mash by Aspergillus aculeatinus mutant enzyme cocktail. Biomass Conv. Bioref. 13, 11803–11812 (2023). https://doi.org/10.1007/s13399-021-02221-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02221-2