Abstract
Microalgae are an alternative source of renewable energy and high-value products for pharmaceutical, nutraceutical, etc., due to rich in carbohydrates, proteins, lipids, and high-density lipoproteins. Existing methods for cell disruption and extraction are costly and suffered from low proficiencies. Ionic liquids are proven to be an environmentally friendly substitute to conventional volatile organic solvents. They have been used in extracting different types of biomass, including microalgae. This article reviews the potential of ILs in extracting biomolecules, lipid, and omega-3, from microalgae biomass. The physicochemical properties of ILs, including viscosity, density, and melting point, their advantages and limitation, as well as toxicity and recyclability of ILs in lipid processing, are discussed.
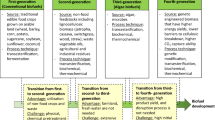
Graphical abstract


reproduced with permission from Taylor and Francis [15])

source of “Sciencedirect” in 5 March 2021)

reproduced with permission from Elsevier [65])

Copyright Springer




Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Zhang S, Liu Z (2021) Advances in the biological fixation of carbon dioxide by microalgae. J Chem Technol Biotechnol 96(6):1475–1495
Aratboni HA, Rafiei N, Garcia-Granados R, Alemzadeh A, Morones-Ramírez JR (2019) Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb Cell Fact 18(1):1–17
Chanthawong A, Dhakal S (2016) Stakeholders’ perceptions on challenges and opportunities for biodiesel and bioethanol policy development in Thailand. Energy Policy 91:189–206
Pan Y et al (2017) One-step production of biodiesel from wet and unbroken microalgae biomass using deep eutectic solvent. Bioresour Technol 238:157–163
A. H. Hirani, N. Javed, M. Asif, S. K. Basu, and A. Kumar, “A review on first-and second-generation biofuel productions,” in Biofuels: Greenhouse Gas Mitigation and Global Warming, Springer, 2018, pp. 141–154.
Lu W, Alam MA, Pan Y, Wu J, Wang Z, Yuan Z (2016) A new approach of microalgal biomass pretreatment using deep eutectic solvents for enhanced lipid recovery for biodiesel production. Bioresour Technol 218:123–128
M. A. Alam, J. Wu, J. Xu, and Z. Wang, “Enhanced isolation of lipids from microalgal biomass with high water content for biodiesel production,” Bioresour. Technol., vol. 291, p. 121834, 2019.
Orr VCA, Rehmann L (2016) Ionic liquids for the fractionation of microalgae biomass. Curr Opin Green Sustain Chem 2(October):22–27. https://doi.org/10.1016/j.cogsc.2016.09.006
D. K. Y. Lim et al., “Isolation and evaluation of oil-producing microalgae from subtropical coastal and brackish waters,” PLoS One, vol. 7, no. 7, p. e40751, 2012.
A. Kumar and J. S. Singh, “Microalgal bio-fertilizers,” in Handbook of Microalgae-Based Processes and Products, Elsevier, 2020, pp. 445–463.
K. M. Rahman, “Food and high value products from microalgae: market opportunities and challenges,” in Microalgae biotechnology for food, health and high value products, Springer, 2020, pp. 3–27.
G. F. Ferreira, L. F. R. Pinto, R. Maciel Filho, and L. V Fregolente, “A review on lipid production from microalgae: association between cultivation using waste streams and fatty acid profiles,” Renew. Sustain. Energy Rev., vol. 109, pp. 448–466, 2019.
Martínez-Francés E, Escudero-Oñate C (2018) Cyanobacteria and microalgae in the production of valuable bioactive compounds. Microalgal Biotechnol 6:104–128
Wang L, Chen L, Yang S, Tan X (2020) Photosynthetic conversion of carbon dioxide to oleochemicals by cyanobacteria: Recent advances and future perspectives. Front Microbiol 11:634
Odjadjare EC, Mutanda T, Olaniran AO (2017) Potential biotechnological application of microalgae: a critical review. Crit Rev Biotechnol 37(1):37–52
C. V. G. López, M. del C. C. García, F. G. A. Fernández, C. S. Bustos, Y. Chisti, and J. M. F. Sevilla, “Protein measurements of microalgal and cyanobacterial biomass,” Bioresour. Technol., vol. 101, no. 19, pp. 7587–7591, 2010.
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25(2):207–210
Lim AS, Jeong HJ, Kim SJ, Ok JH (2018) Amino acids profiles of six dinoflagellate species belonging to diverse families: possible use as animal feeds in aquaculture. Algae 33(3):279–290
Kent M, Welladsen HM, Mangott A, Li Y (2015) Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS One 10(2):e0118985
Tibbetts SM, Milley JE, Lall SP (2015) Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J Appl Phycol 27(3):1109–1119
da Silva Vaz B, Moreira JB, de Morais MG, Costa JAV (2016) Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci 7:73–77
S. R. Motlagh, A. A. Elgharbawy, R. Khezri, R. Harun, and R. Omar, “Ionic liquid-based microwave-assisted extraction of protein from Nannochloropsis sp. biomass,” Biomass Convers. Biorefinery, pp. 1–12, 2021.
Matos J, Cardoso C, Bandarra NM, Afonso C (2017) Microalgae as healthy ingredients for functional food: a review. Food Funct 8(8):2672–2685
Barkia I, Saari N, Manning SR (2019) Microalgae for high-value products towards human health and nutrition. Mar Drugs 17(5):304
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: A review. Renew Sustain Energy Rev 14(1):217–232. https://doi.org/10.1016/j.rser.2009.07.020
Ullah K et al (2015) Assessing the potential of algal biomass opportunities for bioenergy industry: a review. Fuel 143:414–423
Khoo KS et al (2020) Recent advances in downstream processing of microalgae lipid recovery for biofuel production. Bioresour. Technol. 304:122996
S. F. Ahmed et al., “Progress and challenges of contaminate removal from wastewater using microalgae biomass,” Chemosphere, p. 131656, 2021.
Abo BO, Odey EA, Bakayoko M, Kalakodio L (2019) Microalgae to biofuels production: a review on cultivation, application and renewable energy. Rev Environ Health 34(1):91–99
Shahid A et al (2020) Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci. Total Environ 704:135303
Correa DF, Beyer HL, Possingham HP, Fargione JE, Hill JD, Schenk PM (2021) Microalgal biofuel production at national scales: reducing conflicts with agricultural lands and biodiversity within countries. Energy 215:119033
Chen Y, Xu C, Vaidyanathan S (2018) Microalgae: a robust ‘green bio-bridge’ between energy and environment. Crit Rev Biotechnol 38(3):351–368
B. Molinuevo-Salces, B. Riaño, D. Hernández, and M. C. García-González, “Microalgae and wastewater treatment: advantages and disadvantages,” in Microalgae biotechnology for development of biofuel and wastewater treatment, Springer, 2019, pp. 505–533.
S. Rezaei Motlagh et al., “Ionic liquid-based microwave-assisted extraction of lipid and eicosapentaenoic acid from Nannochloropsis oceanica biomass: experimental optimization approach,” J. Appl. Phycol., 2021, https://doi.org/10.1007/s10811-021-02437-9.
Kumar AN et al (2020) Deoiled algal biomass derived renewable sugars for bioethanol and biopolymer production in biorefinery framework. Bioresour. Technol. 296:122315
Lupette J, Benning C (2020) Human health benefits of very-long-chain polyunsaturated fatty acids from microalgae. Biochimie 178:15–25
Raheem A, Wan Azlina WAKG, Taufiq Yap YH, Danquah MK, Harun R (2015) Thermochemical conversion of microalgal biomass for biofuel production. Renew. Sustain. Energy Rev. 49:990–999. https://doi.org/10.1016/j.rser.2015.04.186
Patel A, Mikes F, Matsakas L (2018) An overview of current pretreatment methods used to improve lipid extraction from oleaginous microorganisms. Molecules 23(7):1562
Boni J, Aida S, Leila K (2018) Lipid extraction method from microalgae Botryococcus braunii as raw material to make biodiesel with Soxhlet extraction. J Phys: Conf Ser 1095(1):12004
Breil C, AbertVian M, Zemb T, Kunz W, Chemat F (2017) Bligh and Dyer and Folch methods for solid liquid liquid extraction of lipids from microorganisms. Comprehension of solvatation mechanisms and towards substitution with alternative solvents. Int. J. Mol. Sci. 18(4):1–21. https://doi.org/10.3390/ijms18040708
Y.-H. Tseng, S. K. Mohanty, J. D. McLennan, and L. F. Pease III, “Algal lipid extraction using confined impinging jet mixers,” Chem. Eng. Sci. X, vol. 1, p. 100002, 2019.
Mubarak M, Shaija A, Suchithra TV (2015) A review on the extraction of lipid from microalgae for biodiesel production. Algal Res 7(November):117–123. https://doi.org/10.1016/j.algal.2014.10.008
Barba FJ, Grimi N, Vorobiev E (2015) New approaches for the use of non-conventional cell disruption technologies to extract potential food additives and nutraceuticals from microalgae. Food Eng Rev 7(1):45–62
Saini RK, Keum Y-S (2018) Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—a review. Life Sci 203:255–267
F. Shahidi and P. Ambigaipalan, “Omega-3 polyunsaturated fatty acids and their health benefits,” Annu. Rev. Food Sci. Technol., vol. 9, no. 1, p. annurev-food-111317–095850, 2018, doi: https://doi.org/10.1146/annurev-food-111317-095850.
Choi S-A, Oh Y-K, Jeong M-J, Kim SW, Lee J-S, Park J-Y (2014) Effects of ionic liquid mixtures on lipid extraction from Chlorella vulgaris. Renew Energy 65:169–174
Qv X, Zhou Q, Jiang J (2014) Ultrasound-enhanced and microwave-assisted extraction of lipid from Dunaliella tertiolecta and fatty acid profile analysis. J Sep Sci 37(20):2991–2999
S. P. M. Ventura et al., “Extraction of value-added compounds from microalgae,” in Microalgae-based biofuels and bioproducts, Elsevier, 2017, pp. 461–483.
Kayathi A, Chakrabarti PP, Bonfim-Rocha L, Cardozo-Filho L, Jegatheesan V (2020) Selective extraction of polar lipids of mango kernel using Supercritical carbon dioxide (SC–CO2) extraction: process optimization of extract yield/phosphorous content and economic evaluation. Chemosphere 260:127639
H. Hadiyanto and S. Suttrisnorhadi, “Response surface optimization of ultrasound assisted extraction (UAE) of phycocyanin from microalgae Spirulina platensis,” Emirates J. Food Agric., pp. 227–234, 2016.
Sen Tan J et al (2020) A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 11(1):116–129
Zghaibi N, Omar R, Kamal SMM, Biak DRA, Harun R (2019) Microwave-Assisted Brine Extraction for Enhancement of the Quantity and Quality of Lipid Production from Microalgae Nannochloropsis sp.. Molecules 24(19):3581
Zheng H, Yin J, Gao Z, Huang H, Ji X, Dou C (2011) Disruption of Chlorella vulgaris cells for the release of biodiesel-producing lipids: a comparison of grinding, ultrasonication, bead milling, enzymatic lysis, and microwaves. Appl Biochem Biotechnol 164(7):1215–1224
Wahidin S, Idris A, Shaleh SRM (2014) Rapid biodiesel production using wet microalgae via microwave irradiation. Energy Convers Manag 84:227–233
Lee J-Y, Yoo C, Jun S-Y, Ahn C-Y, Oh H-M (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101(1):S75–S77
Iqbal J, Theegala C (2013) Microwave assisted lipid extraction from microalgae using biodiesel as co-solvent. Algal Res 2(1):34–42
Hernández D, Solana M, Riaño B, García-González MC, Bertucco A (2014) Biofuels from microalgae: lipid extraction and methane production from the residual biomass in a biorefinery approach. Bioresour Technol 170:370–378
Tang S, Qin C, Wang H, Li S, Tian S (2011) Study on supercritical extraction of lipids and enrichment of DHA from oil-rich microalgae. J Supercrit Fluids 57(1):44–49. https://doi.org/10.1016/j.supflu.2011.01.010
Calla-Quispe E, Robles J, Areche C, Sepulveda B (2020) Are ionic liquids better extracting agents than toxic volatile organic solvents? A combination of ionic liquids, microwave and LC/MS/MS, applied to the lichen Stereocaulon glareosum. Front Chem 8:450
M. A. Alam et al., “Choline chloride-based deep eutectic solvents as green extractants for the isolation of phenolic compounds from biomass,” J. Clean. Prod., p. 127445, 2021.
Egorova KS, Gordeev EG, Ananikov VP (2017) Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem Rev 117(10):7132–7189
Vanda H, Dai Y, Wilson EG, Verpoorte R, Choi YH (2018) Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Comptes Rendus Chim 21(6):628–638
Claus J, Sommer FO, Kragl U (2018) Ionic liquids in biotechnology and beyond. Solid State Ionics 314:119–128
A. Basaiahgari and R. L. Gardas, “Ionic Liquids based Aqueous Biphasic Systems as Sustainable Extraction and Separation Techniques,” Curr. Opin. Green Sustain. Chem., p. 100423, 2020.
Sahrash R, Siddiqa A, Razzaq H, Iqbal T, Qaisar S (2018) PVDF based ionogels: applications towards electrochemical devices and membrane separation processes. Heliyon 4(11):e00847
Singh SK, Savoy AW (2020) Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 297:112038
Gomes JM, Silva SS, Reis RL (2019) Biocompatible ionic liquids: fundamental behaviours and applications. Chem Soc Rev 48(15):4317–4335
Marrucho IM, Branco LC, Rebelo LPN (2014) Ionic liquids in pharmaceutical applications. Annu Rev Chem Biomol Eng 5:527–546
Shukla SK, Khokarale SG, Bui TQ, Mikkola J-PT (2019) Ionic liquids: Potential materials for carbon dioxide capture and utilization. Front Mater 6:42
C. Iojoiu, O. Danyliv, and F. Alloin, “Ionic liquids and polymers for battery and fuel cells,” in Modern Synthesis Processes and Reactivity of Fluorinated Compounds, Elsevier, 2017, pp. 465–497.
Floris B, Sabuzi F, Galloni P, Conte V (2017) The beneficial sinergy of MW irradiation and ionic liquids in catalysis of organic reactions. Catalysts 7(9):261
K. S. Khoo et al., “How does ionic liquid play a role in sustainability of biomass processing?,” J. Clean. Prod., p. 124772, 2020.
Troter DZ, Todorović ZB, Đokić-Stojanović DR, Stamenković OS, Veljković VB (2016) Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew Sustain Energy Rev 61:473–500. https://doi.org/10.1016/j.rser.2016.04.011
Alviz PLA, Alvarez AJ (2017) Comparative life cycle assessment of the use of an ionic liquid ([Bmim] Br) versus a volatile organic solvent in the production of acetylsalicylic acid. J Clean Prod 168:1614–1624
Cho HS, Oh YK, Park SC, Lee JW, Park JY (2013) Effects of enzymatic hydrolysis on lipid extraction from Chlorella vulgaris. Renew Energy 54:156–160. https://doi.org/10.1016/j.renene.2012.08.031
Kim Y-H et al (2012) Ionic liquid-mediated extraction of lipids from algal biomass. Bioresour Technol 109:312–315
Cheong L-Z, Guo Z, Yang Z, Chua S-C, Xu X (2011) Extraction and enrichment of n-3 polyunsaturated fatty acids and ethyl esters through reversible π–π complexation with aromatic rings containing ionic liquids. J Agric Food Chem 59(16):8961–8967
Anand M, Hadfield M, Viesca JL, Thomas B, Hernández Battez A, Austen S (2015) Ionic liquids as tribological performance improving additive for in-service and used fully-formulated diesel engine lubricants. Wear 334–335:67–74. https://doi.org/10.1016/j.wear.2015.01.055
Jia X, Han Y, Liu X, Duan T, Chen H (2011) “Speciation of mercury in water samples by dispersive liquid-liquid microextraction combined with high performance liquid chromatography-inductively coupled plasma mass spectrometry”, Spectrochim. Acta - Part B At Spectrosc 66(1):88–92. https://doi.org/10.1016/j.sab.2010.12.003
Qiu Z, Aita GM, Walker MS (2012) Effect of ionic liquid pretreatment on the chemical composition, structure and enzymatic hydrolysis of energy cane bagasse. Bioresour Technol 117:251–256. https://doi.org/10.1016/j.biortech.2012.04.070
Krishnan S et al (2020) Microwave-assisted lipid extraction from Chlorella vulgaris in water with 0.5%–2.5% of imidazolium based ionic liquid as additive. Renew Energy 149:244–252. https://doi.org/10.1016/j.renene.2019.12.063
Wei M, Wang J (2015) A novel acetylcholinesterase biosensor based on ionic liquids-AuNPs-porous carbon composite matrix for detection of organophosphate pesticides. Sensors Actuators B Chem 211:290–296
de Almeida TS, Caparica R, Júlio A, Reis CP (2021) An Overview on Ionic Liquids: A New Frontier for Nanopharmaceuticals. Nanopharmaceuticals Princ Appl 1:181–204
Ventura SPM, Silva FAE, Quental MV, Mondal D, Freire MG, Coutinho JAP (2017) Ionic-liquid-mediated extraction and separation processes for bioactive compounds: past, present, and future trends. Chem. Rev. 117(10):6984–7052
Bystrzanowska M, Pena-Pereira F, Marcinkowski Ł, Tobiszewski M (2019) How green are ionic liquids?–A multicriteria decision analysis approach. Ecotoxicol Environ Saf 174:455–458
P. Lozano, Sustainable catalysis in ionic liquids. CRC Press, 2018.
F. J. V Gschwend, A. Brandt, C. L. Chambon, W.-C. Tu, L. Weigand, and J. P. Hallett, “Pretreatment of lignocellulosic biomass with low-cost ionic liquids,” J. Vis. Exp. JoVE, no. 114, 2016.
Rocha EGA, Pin TC, Rabelo SC, Costa AC (2017) Evaluation of the use of protic ionic liquids on biomass fractionation. Fuel 206:145–154
George A et al (2015) Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem 17(3):1728–1734
Gräsvik J, Winestrand S, Normark M, Jönsson LJ, Mikkola J-P (2014) Evaluation of four ionic liquids for pretreatment of lignocellulosic biomass. BMC Biotechnol 14(1):1–11
Weigand L, Mostame S, Brandt-Talbot A, Welton T, Hallett JP (2017) Effect of pretreatment severity on the cellulose and lignin isolated from Salix using ionoSolv pretreatment. Faraday Discuss 202:331–349
Yang S, Lu X, Zhang Y, Xu J, Xin J, Zhang S (2018) Separation and characterization of cellulose I material from corn straw by low-cost polyhydric protic ionic liquids. Cellulose 25(6):3241–3254
Liu Z, Li L, Liu C, Xu A (2018) Pretreatment of corn straw using the alkaline solution of ionic liquids. Bioresour Technol 260:417–420
Fujita K, Kobayashi D, Nakamura N, Ohno H (2013) Direct dissolution of wet and saliferous marine microalgae by polar ionic liquids without heating. Enzyme Microb Technol 52(3):199–202
Young G, Nippen F, Titterbrandt S, Cooney MJ (2011) Direct transesterification of biomass using an ionic liquid co-solvent system. Biofuels 2(3):261–266
Pan J et al (2016) Microwave-assisted extraction of lipids from microalgae using an ionic liquid solvent [BMIM][HSO4]. Fuel 178:49–55. https://doi.org/10.1016/j.fuel.2016.03.037
Cooney M, Young G, Nagle N (2009) “Separation & Purification Reviews Extraction of Bio - oils from Microalgae.” Sep. Purif. Reveiws 38(June 2013):291–325
Moyer P et al (2018) Relationship between lignocellulosic biomass dissolution and physicochemical properties of ionic liquids composed of 3-methylimidazolium cations and carboxylate anions. Phys Chem Chem Phys 20(4):2508–2516
RezaeiMotlagh S et al (2019) Screening of suitable ionic liquids as green solvents for extraction of eicosapentaenoic acid EPA from microalgae biomass using COSMO RS model. Molecules 24:713. https://doi.org/10.3390/molecules24040713
Rashid Z, Wilfred CD, Gnanasundaram N, Arunagiri A, Murugesan T (2018) Screening of ionic liquids as green oilfield solvents for the potential removal of asphaltene from simulated oil: COSMO-RS model approach. J Mol Liq 255:492–503. https://doi.org/10.1016/j.molliq.2018.01.023
RezaeiMotlagh S et al (2020) COSMO-RS based prediction for alpha-linolenic acid (ALA) extraction from microalgae biomass using room temperature ionic liquids (RTILs). Mar. Drugs 18(2):108
RezaeiMotlagh S et al (2020) Prediction of Potential Ionic Liquids (ILs) for the Solid-Liquid Extraction of Docosahexaenoic Acid (DHA) from Microalgae Using COSMO-RS Screening Model. Biomolecules 10(8):1149
H. W. Khan, A. V. B. Reddy, M. M. E. Nasef, M. A. Bustam, M. Goto, and M. Moniruzzaman, “Screening of ionic liquids for the extraction of biologically active compounds using emulsion liquid membrane: COSMO-RS prediction and experiments,” J. Mol. Liq., p. 113122, 2020.
Lotfi M et al (2017) Solubility of acyclovir in nontoxic and biodegradable ionic liquids: COSMO-RS prediction and experimental verification. J Mol Liq 243:124–131
Passos H, Dinis TBV, Cláudio AFM, Freire MG, Coutinho JAP (2018) Hydrogen bond basicity of ionic liquids and molar entropy of hydration of salts as major descriptors in the formation of aqueous biphasic systems. Phys Chem Chem Phys 20(20):14234–14241
Kurnia KA, Lima F, Cláudio AFM, Coutinho JAP, Freire MG (2015) Hydrogen-bond acidity of ionic liquids: an extended scale. Phys Chem Chem Phys 17(29):18980–18990
Cláudio AFM, Swift L, Hallett JP, Welton T, Coutinho JAP, Freire MG (2014) Extended scale for the hydrogen-bond basicity of ionic liquids. Phys Chem Chem Phys 16(14):6593–6601
Muhammad N et al (2017) Investigation of ionic liquids as a pretreatment solvent for extraction of collagen biopolymer from waste fish scales using COSMO-RS and experiment. J Mol Liq 232:258–264
Mäki-Arvela P, Anugwom I, Virtanen P, Sjöholm R, Mikkola J-P (2010) Dissolution of lignocellulosic materials and its constituents using ionic liquids—a review. Ind Crops Prod 32(3):175–201
Elgharbawy AAM, Hayyan M, Hayyan A, Basirun WJ, Salleh HM, Mirghani MES (2020) A grand avenue to integrate deep eutectic solvents into biomass processing. Biomass and Bioenergy 137:105550
Zubeltzu J, Formoso E, Rezabal E (2020) Lignin solvation by ionic liquids: The role of cation. J. Mol. Liq. 303:112588
Abe M, Kuroda K, Sato D, Kunimura H, Ohno H (2015) Effects of polarity, hydrophobicity, and density of ionic liquids on cellulose solubility. Phys Chem Chem Phys 17(48):32276–32282
Gericke M, Fardim P, Heinze T (2012) Ionic liquids—promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 17(6):7458–7502
Koi ZK, Yahya WZN, Talip RAA, Kurnia KA (2019) Prediction of the viscosity of imidazolium-based ionic liquids at different temperatures using the quantitative structure property relationship approach. New J Chem 43(41):16207–16217
Andanson J-M, Bordes E, Devémy J, Leroux F, Pádua AAH, Gomes MFC (2014) Understanding the role of co-solvents in the dissolution of cellulose in ionic liquids. Green Chem 16(5):2528–2538
A. E. Visser, W. M. Reichert, R. P. Swatloski, H. D. Willauer, J. G. Huddleston, and R. D. Rogers, “Characterization of hydrophilic and hydrophobic ionic liquids: alternatives to volatile organic compounds for liquid-liquid separations,” ACS Publications, 2002.
Zhang J, Wu J, Yu J, Zhang X, He J, Zhang J (2017) Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: state of the art and future trends. Mater Chem Front 1(7):1273–1290
Orr VCA, Plechkova NV, Seddon KR, Rehmann L (2016) Disruption and Wet Extraction of the Microalgae Chlorella vulgaris Using Room-Temperature Ionic Liquids. ACS Sustain Chem Eng 4(2):591–600. https://doi.org/10.1021/acssuschemeng.5b00967
Olkiewicz M et al (2015) A novel recovery process for lipids from microalgæ for biodiesel production using a hydrated phosphonium ionic liquid. Green Chem 17(5):2813–2824
Prabakaran P, Ravindran AD (2011) A comparative study on effective cell disruption methods for lipid extraction from microalgae. Lett Appl Microbiol 53(2):150–154
Young G, Nippgen F, Titterbrandt S, Cooney MJ (2010) Lipid extraction from biomass using co-solvent mixtures of ionic liquids and polar covalent molecules. Sep Purif Technol 72(1):118–121. https://doi.org/10.1016/j.seppur.2010.01.009
Kim YH et al (2013) Ultrasound-assisted extraction of lipids from Chlorella vulgaris using [Bmim][MeSO4]. Biomass Bioenerg 56:99–103. https://doi.org/10.1016/j.biombioe.2013.04.022Shortcommunication
Shankar M, Chhotaray PK, Agrawal A, Gardas RL, Tamilarasan K, Rajesh M (2017) Protic ionic liquid-assisted cell disruption and lipid extraction from fresh water Chlorella and Chlorococcum microalgae. Algal Res 25:228–236
W. Zhou et al., “Repeated utilization of ionic liquid to extract lipid from algal biomass,” Int. J. Polym. Sci., vol. 2019, 2019.
Choi S-A, Lee J-S, Oh Y-K, Jeong M-J, Kim SW, Park J-Y (2014) Lipid extraction from Chlorella vulgaris by molten-salt/ionic-liquid mixtures. Algal Res 3:44–48
Cardoso-Ugarte GA, Juárez-Becerra GP, SosaMorales ME, López-Malo A (2013) Microwave-assisted extraction of essential oils from herbs. J Microw Power Electromagn Energy 47(1):63–72
Wahidin S, Idris A, Shaleh SRM (2016) Ionic liquid as a promising biobased green solvent in combination with microwave irradiation for direct biodiesel production. Bioresour Technol 206:150–154. https://doi.org/10.1016/j.biortech.2016.01.084
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv 30(3):709–732
D. O. Hartmann and C. S. Pereira, “Toxicity of ionic liquids: past, present, and future,” in Ionic Liquids in Lipid Processing and Analysis, Elsevier, 2016, pp. 403–421.
Huang W et al (2020) Ionic liquids: Green and tailor-made solvents in drug delivery. Drug Discov Today 25(5):901–908
Mezzetta A, Guazzelli L, Seggiani M, Pomelli CS, Puccini M, Chiappe C (2017) A general environmentally friendly access to long chain fatty acid ionic liquids (LCFA-ILs). Green Chem 19(13):3103–3111
Phuong T, Pham T, Cho C, Yun Y (2010) Environmental fate and toxicity of ionic liquids : A review. Water Res 44(2):352–372. https://doi.org/10.1016/j.watres.2009.09.030
M. Cvjetko, K. Rado, I. Radoj, and J. Halambek, “Ecotoxicology and Environmental Safety A brief overview of the potential environmental hazards of ionic liquids,” vol. 99, pp. 1–12, 2014, doi: https://doi.org/10.1016/j.ecoenv.2013.10.019.
L. El Blidi, J. Saleh, O. Ben Ghanem, M. El-harbawi, and M. K. Hadj-kali, “Synthesis, Characterization, and Antimicrobial Toxicity Study of Dicyanamide-Based Ionic Liquids and Their Application to Liquid − Liquid Extraction,” 2020, doi: https://doi.org/10.1021/acs.jced.9b00654.
Viboud S, Papaiconomou N, Cortesi A, Chatel G, Draye M, Fontvieille D (2012) Correlating the structure and composition of ionic liquids with their toxicity on Vibrio fischeri: a systematic study. J Hazard Mater 215:40–48
Flieger J, Flieger M (2020) Ionic Liquids Toxicity-Benefits and Threats. Int J Mol Sci 21(17):6267. https://doi.org/10.3390/ijms21176267
Bubalo MC, Radošević K, Redovniković IR, Slivac I, Srček VG (2017) Toxicity mechanisms of ionic liquids. Arch Ind Hyg Toxicol 68(3):171–179
Ma J, Cai L, Zhang B, Hu L, Li X, Wang J (2010) Ecotoxicology and Environmental Safety Acute toxicity and effects of 1-alkyl-3-methylimidazolium bromide ionic liquids on green algae. Ecotoxicol Environ Saf 73(6):1465–1469. https://doi.org/10.1016/j.ecoenv.2009.10.004
Petkovic M et al (2010) Novel biocompatible cholinium-based ionic liquids—toxicity and biodegradability. Green Chem 12(4):643–649
Weyhing-Zerrer N, Kalb R, Oßmer R, Rossmanith P, Mester P (2018) Evidence of a reverse side-chain effect of tris (pentafluoroethyl) trifluorophosphate [FAP]-based ionic liquids against pathogenic bacteria. Ecotoxicol Environ Saf 148:467–472
Steudte S et al (2014) Toxicity and biodegradability of dicationic ionic liquids. RSC Adv 4(10):5198–5205
M. Matzke, S. Stolte, K. Thiele, T. Juffernholz, and J. Ranke, “The influence of anion species on the toxicity of 1-alkyl-3- methylimidazolium ionic liquids observed in an ( eco ) toxicological test battery {,” pp. 1198–1207, 2007, doi: https://doi.org/10.1039/b705795d.
Vieira NSM, Stolte S, Araújo JMM, Rebelo LPN, Pereiro AB, Markiewicz M (2019) Acute aquatic toxicity and biodegradability of fluorinated ionic liquids. ACS Sustain Chem Eng 7(4):3733–3741
Bogdanov MG, Svinyarov I (2018) Analysis of acetylcholinesterase inhibitors by extraction in choline saccharinate aqueous biphasic systems. J Chromatogr A 1559:62–68
Sivapragasam M, Wilfred CD, Jaganathan JR, Krishnan S, WanKarim Ghani WAWAB (2019) Choline-Based Ionic Liquids as Media for the Growth of Saccharomyces cerevisiae. Processes 7(7):471
Santos JI et al (2015) Environmental safety of cholinium-based ionic liquids: assessing structure–ecotoxicity relationships. Green Chem 17(9):4657–4668
Petkovic M, Seddon KR, Rebelo LPN, Pereira CS (2011) Ionic liquids: a pathway to environmental acceptability. Chem Soc Rev 40(3):1383–1403
Zhang S et al (2018) The ecotoxicity and tribological properties of choline amino acid ionic liquid lubricants. Tribol Int 121:435–441
Neves CMSS et al (2014) The impact of ionic liquid fluorinated moieties on their thermophysical properties and aqueous phase behaviour. Phys Chem Chem Phys 16(39):21340–21348
C. Cho, Y. Jeon, T. Phuong, T. Pham, K. Vijayaraghavan, and Y. Yun, “The ecotoxicity of ionic liquids and traditional organic solvents on microalga Selenastrum capricornutum $,” vol. 71, pp. 166–171, 2008, doi: https://doi.org/10.1016/j.ecoenv.2007.07.001.
Stolte S et al (2007) Effects of different head groups and functionalised side chains on the cytotoxicity of ionic liquids. Green Chem 9(7):760–767
R. P. Swatloski et al., “Using Caenorhabditis elegans to probe toxicity of 1-alkyl-3-methylimidazolium chloride based ionic liquids,” pp. 668–669, 2004, doi: https://doi.org/10.1016/S0147-6513(03)00105-2.
P. Attri, P. M. Reddy, and P. Venkatesu, “Density and ultrasonic sound speed measurements for N, N-dimethylformamide with ionic liquids,” 2010.
Zhang Y et al (2018) Optimization of enzymatic hydrolysis for effective lipid extraction from microalgae Scenedesmus sp. Renew Energy 125:1049–1057
Acknowledgements
The authors are grateful to the Department of Chemical and Environmental Engineering, Universiti Putra Malaysia (UPM).
Author information
Authors and Affiliations
Contributions
Conceptualization of the review was proposed by S.R.M. and A.A.E.; writing (original draft preparation) was done by S.R.M. and A.A.E.; final review and editing were done by S.R.M., R.K., and A.A.E; project supervision was by R.H., D.R.A.B., and S.A.H.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Motlagh, S.R., Elgharbawy, A.A., khezri, R. et al. Ionic liquid method for the extraction of lipid from microalgae biomass: a review. Biomass Conv. Bioref. 13, 11417–11439 (2023). https://doi.org/10.1007/s13399-021-01972-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01972-2