Abstract
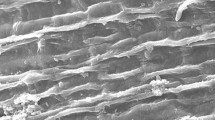
New sorbent was prepared from unicellular green microalgal Chlorella pyrenoidosa (CP) immobilized in Luffa cylindrica (CP-LC) as a biosorbent for the removal of lead ions from aqueous media in a batch experiment mode. The prepared biosorbent was characterized by Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM). In the experimental design of the Pb (II) removal process, the equilibrium isotherms were studied and modeled. To select the best-fit isotherm model for this biological process, the experimental data were fitted in the eighteen different types of one-, two-, three-, four-, and five-parameter isotherm models. A comparison of non-linear models for selecting the optimum isotherm showed Fritz–Schlunder (V) model gives the most accurate fit to describe the experimental data, determined based on several error functions. Taking into account the outcome and in an attempt to optimize the equilibrium isotherms, the equation of Fritz-Schlünder (V) was modified to obtain more sophisticated model and more precision parameters values. As a consequence of such a developed model to existing mentioned ones, the mathematical model developed in this study is more suitable for predicting the equilibrium data. According to the results obtained, the evaluation of experimental data in terms of biosorption kinetics elucidated that the biosorption of Pb (II) by CP-LC well followed pseudo-second-order kinetics. The maximum biosorbent capacity of CP-LC was found to be 123 mg/g for 40 min at pH5. The calculated thermodynamic factors (∆G°, ∆H°, and ∆S°) indicated that the biosorption process was favorable, spontaneous, and exothermic at 298–318 K.










Similar content being viewed by others
Abbreviations
- Ak (mg/g):
-
Koble-Carrigan isotherm constant
- ae (-):
-
Activity of Pb (II) in solution at equilibrium
- akh (–):
-
Constant in Khan isotherm
- aR (L/g):
-
Constant in Redlich–Peterson isotherm
- ARE:
-
average relative error
- A T (L/g):
-
Equilibrium binding constant
- as:
-
Activity of adsorbed Pb (II)
- bo (L/g):
-
Constant in Baudu isotherm
- Bk (L/g):
-
Koble-Carrigan isotherm constant
- bKH (L/g):
-
Constant in Khan isotherm
- bR (–):
-
Constant in Redlich–Peterson isotherm
- BT (Kj/mol):
-
Temkin constant
- b T (Kj/mol):
-
Adsorption energy
- C (L/g):
-
Fritz-Schlünder (IV) constant
- C0 (mg/L):
-
Initial Pb (II) ion concentration
- Ce (mg/L):
-
Pb (II) ion concentration in solution at equilibrium
- Cs (mg/g):
-
Pb (II) adsorbed on CP-LC
- CP:
-
Chlorella pyrenoidosa
- CP-LC:
-
Chlorella pyrenoidosa immobilized in luffa cylindrica
- D (L/g):
-
Fritz-Schlünder (IV) constant
- ERSQ (–):
-
Sum of the squares of the errors
- GM:
-
Growth medium
- HYBRID:
-
Hybrid fractional error function
- IUPAC:
-
International union of pure and applied chemistry
- K (–):
-
Henry’s isotherm model adsorption constant
- K1 (1/min):
-
The rate constant of pseudo first-order model
- K2 (g/mg/min):
-
the rate constant of pseudo second-order model
- Kc (-):
-
The distribution constant
- K K1 (L/g):
-
Kiselev equilibrium constant
- K K2 (–):
-
Constant of complex formation between adsorbed molecules
- KE (L/mg):
-
Elovich constant
- K F (mg/g)(L/g)1/n :
-
Adsorption capacity
- K FH (L/g):
-
Flory–Huggins equilibrium constant
- K FG (L/g):
-
Fowler–Guggenheim equilibrium constant
- KFS1 (L/g):
-
Fritz-Schlünder (v) equilibrium constant
- kFS2 (L/g):
-
Fritz-Schlünder (v) equilibrium constant
- K H (L/g):
-
Hill–de Boer constant
- KL (L/mg):
-
Constant in Langmuir isotherm
- K n (k/J/mol):
-
E nergetic constant of the interaction between adsorbed molecules
- KR (L/g):
-
Constant in Redlich–Peterson isotherm
- KRP (L/g):
-
Constant in Radke–Prausnitz isotherm
- ks (L/g):
-
Constant in Sips isotherm
- KT (L/g):
-
Constant in Toth isotherm
- m (g/L):
-
Dose of free or immobilized cells
- M1, M2 (–):
-
Constants in Fritz-Schlünder model
- MPSD:
-
Marquardt’s percent standard deviation
- N (–):
-
Number of observations in the experimental isotherm
- n (–):
-
Constant in Freundlich isotherm
- n FH (–):
-
Number of adsorbates occupying adsorption sites
- nK (–):
-
Noefficient in Koble-Carrigan isotherm
- nR (–):
-
Constant in Radke–Prausnitz isotherm
- nS (–):
-
Coefficient in Sips isotherm
- nT (–):
-
Constant in Toth isotherm
- P (–):
-
Number of parameters in the regression model
- qcal (mg/g):
-
Estimate from the isotherm for corresponding qexp
- qe (mg/g):
-
Adsorption capacity
- qexp (mg/g):
-
Observation from the batch experiment
- q mKH (mg/g):
-
Maximum adsorbate uptake from Khan model
- q m (mg/g):
-
Maximum adsorbate uptake from Radke–Prausnitz model
- q mb (mg/g):
-
Maximum adsorbate uptake from Baudu model
- q max (mg/g):
-
Maximum adsorbate uptake from Langmuir model
- q mE (mg/g):
-
Maximum adsorbate uptake from elovich model
- 푞mFS (mg/g):
-
Fritz-Schlünder (v) maximum adsorption capacity.
- q mk (mg/g):
-
Maximum adsorbate uptake from Toth model
- q mS (mg/g):
-
Maximum adsorbate uptake from sips model
- R (J/mol K):
-
Ideal gas constant
- R2 (–):
-
Coefficient of determination
- RL (-):
-
Separation factor
- S2 :
-
Residual variance
- SEM:
-
Scaninig electron microscopy
- t (min):
-
Time
- T (K):
-
Temperature
- W (KJ/mol):
-
Interaction energy between adsorbed molecules
- x, y (–):
-
Constants in Baudu isotherm
- Ye (–):
-
Activity coefficient of Pb (II) in equilibrium solution
- Ys (–):
-
Activity coefficient of adsorbed Pb (II)
- ∆H◦ (kJ/mol):
-
Enthalpy change
- ∆S◦ (J/mol K):
-
Entropy change
- ∆G◦(kJ/mol):
-
Gibbs free energy change (kJ/mol)
- Ø (–):
-
Surface coverage of the adsorbent
- α, ß (–):
-
Constant in modified Fritz-Schlunder (v)
References
Wei BG, Yang LS (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107. https://doi.org/10.1016/j.microc.2009.09.014
Wang J, Chen C (2006) Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol Adv 24(5):427–451. https://doi.org/10.1016/j.biotechadv.2006.03.001
Abhari PS, Manteghi F, Tehrani Z (2020) Adsorption of lead ions by a green AC/HKUST-1 nanocomposite. Nanomaterials 10(9):1647. https://doi.org/10.3390/nano10091647
Agarwal A, Upadhyay U, Sreedhar I, Singh SA, Patel CM (2020) A review on valorization of biomass in heavy metal removal from wastewater. J Water Process Eng 38:101602. https://doi.org/10.1016/j.jwpe.2020.101602
Moreira VR, Lebron YAR, Freire SJ, Santos LVS, Palladino F, Jacob RS (2019) Biosorption of copper ions from aqueous solution using Chlorella pyrenoidosa: optimization, equilibrium and kinetics studies. Microchem J 145:119–129. https://doi.org/10.1016/j.microc.2018.10.027
Vidal CB, Melo DQ, Raulino GSC, da Luz AD, da Luz C, Nascimento RF (2016) Multielement adsorption of metal ions using Tururi fibers (Manicaria saccifera): experiments, mathematical modeling and numerical simulation. Desalin Water Treat 57(19):9001–9008. https://doi.org/10.1080/19443994.2015.1025441
Kumar KV (2006) Comparative analysis of linear and non-linear method of estimating the sorption isotherm parameters for malachite green onto activated carbon. J Hazard Mater 136(21):197–202. https://doi.org/10.1016/j.jhazmat.2005.09.018
Kumar KV, Porkodi K (2006) Relation between some two- and three-parameter isotherm models for the sorption of methylene blue onto lemon peel. J Hazard Mater 138:633–635
Haloi N, Sarma HP, Chakravarty P (2013) Biosorption of lead (II) from water using heartwood charcoal of Areca catechu: equilibrium and kinetics studies. Appl Water Sci 3:559–565. https://doi.org/10.1007/s13201-013-0112-3
Kuczajowska-Zadrożna M, Filipkowska U, Jóźwiak T (2020) Adsorption of Cu (II) and Cd (II) from aqueous solutions by chitosan immobilized in alginate beads. J Environ Chem Eng 8:103878. https://doi.org/10.1016/j.jece.2020.103878
Al-Ghouti MA, Da'ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:122383. https://doi.org/10.1016/j.jhazmat.2020.122383
Rangabhashiyam S, Anu N, Giri Nandagopal MS, Selvaraju N (2014) Relevance of isotherm models in biosorption of pollutants by agricultural byproducts. J Environ Chem Eng 2(1):398–414. https://doi.org/10.1016/j.jece.2014.01.014
Bayuo J, Abukari MA, Pelig-Ba KB (2020) Desorption of chromium (VI) and lead (II) ions and regeneration of the exhausted adsorbent. Appl Water Sci 10:171. https://doi.org/10.1007/s13201-020-01250-y
Ding TY, Hii SL, Ong, L (2012) Ong, Comparison of pretreatment strategies for conversion of coconut husk fiber to fermentable sugars. Bioresources 7(2): 1540–1547. https://doi.org/10.15376/biores.7.2.1540-1547.
Rescignano N, Fortunati E, Armentano I, Hernandez R, Mijangos C, Pasquino R, Kenny JM (2015) Use of alginate, chitosan and cellulose nanocrystals as emulsion stabilizers in the synthesis of biodegradable polymeric nanoparticles. J Colloid Interface Sci 445:31–39. https://doi.org/10.1016/j.jcis.2014.12.032
Mitra T, Sailakshmi G, Gnanamani A, Mandal AB (2013) Studies on cross-linking of succinic acid with chitosan/collagen. Mater Res 16:755–765. https://doi.org/10.1590/S1516-14392013005000059
Lim SH, Hudson SM (2004) Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr Res 339:313–319. https://doi.org/10.1016/j.carres.2003.10.024
Bhattacharya A, Mathur M, Kumar P, Malik A (2019) Potential role of N-acetyl glucosamine in Aspergillus fumigatus-assisted Chlorella pyrenoidosa harvesting. Biotechnol Biofuels 12:178. https://doi.org/10.1186/s13068-019-1519-3
Leal D, Matsuhiro B, Rossi M, Caruso F (2008) FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res, 343(2): 308-316. https://doi.org/10.1016/j.carres.2007.10.016.
Taşar Ş, Kaya F, Özer A (2014) Biosorption of lead(II) ions from aqueous solution by peanut shells: equilibrium, thermodynamic and kinetic studies. Journal of Environmental Chemical Engineering. J Environ Chem Eng 2(2):1018–1026. https://doi.org/10.1016/j.jece.2014.03.015
Ozer A (2007) Removal of Pb(II) ions from aqueous solutions by sulphuric acid-treated wheat bran. J Hazard Mater 141(3):753–761. https://doi.org/10.1016/j.jhazmat.2006.07.040
Adewuyi A, Pereira FV (2017) Underutilized Luffa cylindrica sponge: a local bio-adsorbent for the removal of Pb(II) pollutant from water system. Beni Suef Univ J Basic Appl Sci 6(2):118–126. https://doi.org/10.1016/j.bjbas.2017.02.001
Karnib M, Kabbani A, Holail H, Olama,Z (2014) Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia 50: 113–120. doi: https://doi.org/10.1016/j.egypro.2014.06.014.
Ajenifuja E, Ajao JA, Ajayi EOB (2017) Adsorption isotherm studies of Cu (II) and Co (II) in high concentration aqueous solutions on photocatalytically modified diatomaceous ceramic adsorbents. Appl Water Sci 7:3793–3801. https://doi.org/10.1007/s13201
Awwad A M, Salem N M (2014) Kinetics and thermodynamics of Cd(II) biosorption onto loquat (Eriobotrya japonica) leaves. J Saudi Chem Soc 18 (5): 486–493. https://doi.org/10.1016/j.jscs.2011.10.007.
Bhaumik R, Mondal NK (2016) Optimizing adsorption of fluoride from water by modified banana peel dust using response surface modelling approach. Appl Water Sci 6:115–135. https://doi.org/10.1007/s13201-014-0211-9
Farhan SN, Khadom AA (2015) Biosorption of heavy metals from aqueous solutions by Saccharomyces Cerevisiae. Int J Ind Chem 6(2):119–130. https://doi.org/10.1007/s40090-015-0038-8
Wei W, Wang Q, Li A, Yang J, Ma F, Pi S, Wu D (2016) Biosorption of Pb (II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp. J1: adsorption behavior and mechanism assessment. Sci Rep 6:31575. https://doi.org/10.1038/srep31575
Zulkali MMD, Ahmad AI, Norulakmal NH, Sharifah NS (2006) Comparative studies of Oryza sativa L. husk and chitosan as lead adsorbent. J Chem Technol Biotechnol 81:1324–1327. https://doi.org/10.1002/jctb.1429
Sarı A, Tuzen M, Uluözlü ÖD, Soylak M (2007) Biosorption of Pb(II) and Ni(II) from aqueous solution by lichen (Cladonia furcata) biomass. Biochem Eng J 37(2):151–158. https://doi.org/10.1016/j.bej.2007.04.007
Ronda A, Martín-Lara MA, Dionisio E, Blázquez G, Calero M (2013) Effect of lead in biosorption of copper by almond shell. J Taiwan Inst Chem Eng 44(3):466–473. https://doi.org/10.1016/j.jtice.2012.12.019
Mahamadi C, Nharingo T (2010) Competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto Eichhornia crassipes in binary and ternary systems. Bioresour Technol 101(3):859–864. https://doi.org/10.1016/j.biortech.2009.08.097
Gundogdu A, Ozdes D, Duran C, Bulut VN, Soylak M, Senturk HB (2009) Biosorption of Pb(II) ions from aqueous solution by pine bark (Pinus brutia Ten.). Chem Eng Sci 153(1-3):62–69. https://doi.org/10.1016/j.cej.2009.06.017
Naiya TK, Bhattacharya AK, Das SK (2008) Adsorption of Pb(II) by sawdust and neem bark from aqueous solutions. Environ Prog 27(3):313–328. https://doi.org/10.1002/ep.10280
Pavan FA, Mazzocato AC, Jacques RA, Dias SLP (2008) Ponkan peel: a potential biosorbent for removal of Pb(II) ions from aqueous solution. "Biochem. Eng. J 40(2): 357–362. https://doi.org/10.1016/j.bej.2008.01.004.
Acknowledgements
The authors (s) thank the anonymous reviewers for helping us to present this research in a more effective manner. The authors also are thankful to Mr. Ramzi Touchan, Research Professor at the Laboratory of Tree-Ring Research, University of Arizona, for his critical suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nouacer, I., Benalia, M., Henini, G. et al. Mathematical modeling and interpretation of equilibrium isotherms of Pb (II) from aqueous media by Chlorella pyrenoidosa immobilized in Luffa cylindrica. Biomass Conv. Bioref. 13, 7839–7858 (2023). https://doi.org/10.1007/s13399-021-01722-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01722-4