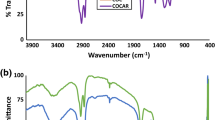
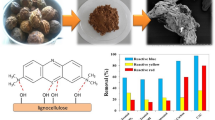
Abstract
Wood-based sorbents chemically modified by Al2O3 (WB–Al2O3) were synthesized via solvo-reaction method. The wood residue, a waste material with lignocellulosic structure, was obtained from Quercus robur (oak tree). WB–Al2O3 sorbents were tested for removal of Reactive Blue 19 (RB19) dye. The influence of solvents with different polarity and protic/aprotic nature used in the sorbent synthesis, such as acetone, ethanol, ether, hexane, and methanol, were investigated and the best sorbent capable to remove the highest percentage of dye was determined. To investigate the reason for difference between dye removal efficiency of the synthesized sorbents, the appropriate characterization of the sorbents was done. The highest dye removal efficiency (100%) and the shortest equilibrium time (less than 10 min) were achieved for the WB–Al2O3 sorbent synthesized in less polar solvent — hexane. The sorption kinetics can be described by the pseudo-second-order model. The equilibrium examination showed that the Langmuir model better explained sorption process and the maximal capacity of the WB–Al2O3 was 441.90 mg/g. The present research shows that usage of nature lignocellulosic polymers in the synthesis of the low economically cost sorbents can effectively decrease the water pollution and increase the reusability of wood biowaste.




Similar content being viewed by others
References
Kaya M (2018) Evaluation of a novel woody waste obtained from tea tree sawdust as an adsorbent for dye removal. Wood Sci Technol 52:245–260. https://doi.org/10.1007/s00226-017-0945-2
Leme DM, de Oliveira GAR, Meireles G, Brito LB, de Rodrigues LB, Palma de Oliveira D (2015) Eco- and genotoxicological assessments of two reactive textile dyes. J Toxicol Environ Health Part A 78:287–300. https://doi.org/10.1080/15287394.2014.971208
Fathy N, El-Shafey SE, El-Shafey OI, Mohamed WS (2013) Oxidative degradation of RB19 dye by a novel γ-MnO2/MWCNT nanocomposite catalyst with H2O2. J Environ Chem Eng 1:858–864. https://doi.org/10.1016/j.jece.2013.07.028
Kadhom M, Albayati N, Alalwan H, Al-Furaiji M (2020) Removal of dyes by agricultural waste. Sustain Chem Pharm 16:100259. https://doi.org/10.1016/j.scp.2020.100259
Monteiro MS, de Faria RF, Chaves JAP, Santana SA, Silva HAS, Bezerra CWB (2017) Wood (Bagassa guianensis Aubl) and green coconut mesocarp (cocos nucifera) residues as textile dye removers (Remazol Red and Remazol Brilliant Violet). J Environ Manage 204:23–30. https://doi.org/10.1016/j.jenvman.2017.08.033
Djilali Y, Elandaloussi EH, Aziz A, de Menorval L-C (2016) Alkaline treatment of timber sawdust: a straightforward route toward effective low-cost adsorbent for the enhanced removal of basic dyes from aqueous solutions. J Saudi Chem Soc 20:S241–S249. https://doi.org/10.1016/j.jscs.2012.10.013
Mondal M, Goswami DK, Bhattacharyya TK (2021) Lignocellulose based bio-waste materials derived activated porous carbon as superior electrode materials for high-performance supercapacitor. J Energy Storage 34:102229. https://doi.org/10.1016/j.est.2020.102229
Shakoor S, Nasar A (2018) Adsorptive decontamination of synthetic wastewater containing crystal violet dye by employing Terminalia arjuna sawdust waste. Groundw Sustain Dev 7:30–38. https://doi.org/10.1016/j.gsd.2018.03.004
Zhang A, Liu J, Zhang Z, Yang Y, Yu Y, Zhao Y (2021) Insights into the mechanism of lead species adsorption over Al2O3 sorbent. J Hazard Mater 413:125371. https://doi.org/10.1016/j.jhazmat.2021.125371
Dang Z, Jia M, Liao J, Zhang Y, Zhu W (2021) Fabrication of the Al2O3 aerogels by in situ water formation method for the highly efficient removal of uranium(VI). Microporous Mesoporous Mater 316:110952. https://doi.org/10.1016/j.micromeso.2021.110952
Yang K, Li Y, Tian Z, Peng K, Lai Y (2020) Removal of fluoride ions from ZnSO4 electrolyte by amorphous porous Al2O3 microfiber clusters: adsorption performance and mechanism. Hydrometallurgy 197:105455. https://doi.org/10.1016/j.hydromet.2020.105455
Kassem Hami H, Fahmi Abbas R, Amer Waheb A, Ibrahim Mahdi N (2020) Removal of Eriochrom Black T from aqueous solution using Al2O3 surface: linear and non-linear isotherm models, error analysis and thermodynamic studies. Mater Today: Proceedings 20:599–604. https://doi.org/10.1016/j.matpr.2019.09.196
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319. https://doi.org/10.1021/ja01269a023
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380. https://doi.org/10.1021/ja01145a126
Kuśmierek K, Świątkowski A (2015) The influence of different agitation techniques on the adsorption kinetics of 4-chlorophenol on granular activated carbon. React Kinet Mech Catal 116:261–271. https://doi.org/10.1007/s11144-015-0889-1
García Díaz I, Alguacil FJ, Escudero E, López FA (2020) Evaluation of La (III) and Ce(III) adsorption from aqueous solution using carbon nanotubes adsorbent. Preprints.org, https://doi.org/10.20944/preprints202001.0254.v1
Kondo, T. Hydrogen bonds in cellulose and cellulose derivatives S. Dumitriu (Ed.), Polysaccharides II-Structural diversity and functional versatility, Marcel Dekker, New York, 2005
Broda M, Popescu C-M (2019) Natural decay of archaeological oak wood versus artificial degradation processes – an FT-IR spectroscopy and X-ray diffraction study. Spectrochim Acta A 209:280–287. https://doi.org/10.1016/j.saa.2018.10.057
Vazquez-Guerrero A, Alfaro-Cuevas-Villanueva R, Rutiaga-Quinones J-G, Cortes-Martinez R (2016) Fluoride removal by aluminum-modified pine sawdust: effect of competitive ions. Ecol Eng 94:365–379. https://doi.org/10.1016/j.ecoleng.2016.05.070
El-Sakhawy M (2001) Characterization of modified oxycellulose. J Therm Anal Cal 63:549–558. https://doi.org/10.1023/A:1010150122848
Gupta M, Singh V, Katyal P (2018) Synthesis and structural characterization of Al2O3 nanofluids. Mater Today: Proceedings 5:27989–27997. https://doi.org/10.1016/j.matpr.2018.10.039
Reichardt C (2003) Solvents and solvent effects in organic chemistry. Wiley-VCH Verlag, Weinheim, Germany
Sadek PC (2002) The HPLC solvent guide. Wiley-Interscience, New York
Degermenci GD, Degermenci N, Ayvaoglu V, Durmaz E, Cakir D, Akan E (2019) Adsorption of reactive dyes on lignocellulosic waste; characterization equilibrium, kinetic and thermodynamic studies. J Clean Prod 225:1220–1229. https://doi.org/10.1016/j.jclepro.2019.03.260
Ogunleye DT, Akpotu SO, Moodley B (2020) Adsorption of sulfamethoxazole and reactive blue 19 using graphene oxide modified with imidazolium based ionic liquid. Environ Technol Inn 17:100616. https://doi.org/10.1016/j.eti.2020.100616
Banaei A, Ebrahimi S, Vojoudi H, Karimi S, Badiei A, Pourbasheer E (2017) Adsorption equilibrium and thermodynamics of anionic reactive dyes from aqueous solutions byusing a new modified silica gel with 2,2′-(pentane-1,5-diylbis(oxy))dibenzaldehyde. Chem Eng Res Des 123:50–62. https://doi.org/10.1016/j.cherd.2017.04.032
Abbasi M (2017) Synthesis and characterization of magnetic nanocomposite of chitosan/SiO2/carbon nanotubes and its application for dyes removal. J Clean Prod 145:105–113. https://doi.org/10.1016/j.jclepro.2017.01.046
Shanehsaz M, Seidi S, Ghorbani Y, Shoja SMR, Rouhani S (2015) Polypyrrole-coated magnetic nanoparticles as an efficient adsorbent for RB19 synthetic textile dye: removal and kinetic study. Spectrochim Acta A Mol Biomol Spectrosc 149:481–486. https://doi.org/10.1016/j.saa.2015.04.114
Gok O, Ozcan AS, Ozcan A (2010) Adsorption behavior of a textile dye of Reactive Blue 19 from aqueous solutions onto modified bentonite. Appl Surf Sci 256:5439–5443. https://doi.org/10.1016/j.apsusc.2009.12.134
Lagergren S (1898) Zur Theorie der sogenannten Adsorption gelöster Stoffe (About the theory of so-called adsorption of soluble substances). Kungliga Svenska Vetenskapsakademiens Handl 24:1–39
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf Environ Prot 76:183–191
Wong S, Tumari HH, Ngadi N, Mohamed NB, Hassan O, Mat R, Amin NAS (2019) Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL). J Clean Prod 206:394–406. https://doi.org/10.1016/j.jclepro.2018.09.201
Sabar S, Abdul Aziz H, Yusof NH, Subramaniam S, Foo KY, Wilson LD, Lee HK (2020) Preparation of sulfonated chitosan for enhanced adsorption of methylene blue from aqueous solution. React Funct Polym 151:104584. https://doi.org/10.1016/j.reactfunctpolym.2020.104584
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich HZ (1906) Over the adsorption in solution. J Phys Chem 57A:385–470
Sips R (1948) On the structure of a catalyst surface. J Chem Phys 16:490–495. https://doi.org/10.1063/1.1746922
Todorciuc T, Bulgariu L, Popa IV (2015) Adsorption of Cu(II) from aqueous solution on wheat straw lignin: equilibrium and kinetic studies. Cell Chem Technol 49:439–447
Sharma PR, Sharma SK, Antoine R, Hsiao BS (2019) Efficient removal of arsenic using zinc oxide nanocrystal-decorated regenerated microfibrillated cellulose scaffolds. ACS Sustainable Chem Eng 7:6140–6151. https://doi.org/10.1021/acssuschemeng.8b06356
Selvi EK, Şahin U, Şahan S (2017) Determination of aluminum in dialysis concentrates by atomic absorption spectrometry after coprecipitation with lanthanum phosphate. Iran J Pharm Res 16:1030–1036
Funding
The authors would like to acknowledge financial support from the Ministry of Education, Science and Technological Development of the Republic of Serbia (Agreement No 451-03-9/2021-14/200124).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Velinov, N., Radović Vučić, M., Petrović, M. et al. The influence of various solvents’ polarity in the synthesis of wood biowaste sorbent: evaluation of dye sorption. Biomass Conv. Bioref. 13, 8139–8150 (2023). https://doi.org/10.1007/s13399-021-01691-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01691-8