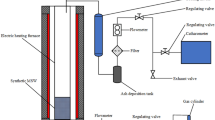
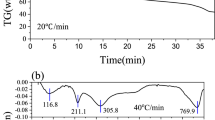
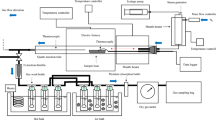
Abstract
Pyrolysis of dry kitchen waste (KW) has been investigated using TGA/DTG, DSC, and lab-scale fixed-bed pyrolyser. Thermal characterization of the dry kitchen waste (KW) was carried out using standard protocols. The pyrolysis has been carried out at three different heating rates of 15, 20, and 40 °C/min from room temperature to 1000 °C. Kinetic analysis of the pyrolysis process using TGA data has been carried using iso-conversional model-free methods of Flynn-Wall-Ozawa (FWO), Kissinger–Akahira–Sunose (KAS), Tang, and Starink methods as well as model fitting method of Coats–Redfern. The pyrolysis for liquid yield (bio-oil) of pelletized KW was also performed at 400, 450, 500, 550, and 600 °C at a single heating rate of 15 °C/min in an inert atmosphere using a lab-scale fixed-bed pyrolyser. The maximum bio-oil yield (29.52%) was obtained at 500 °C. The FTIR results indicated the presence of alcoholic, phenolic, ester, and oxygenated group containing compounds, and GCMS results also indicated the presence of varying amounts of several organic molecules. The calorific value of the bio-oil was found to be 24 MJ/kg.
Graphical abstract







Similar content being viewed by others
Abbreviations
- A:
-
Arrhenius pre-exponential factor, s−1
- C1 :
-
constant
- E:
-
activation energy, kJ/mol
- f(α):
-
reaction model based function of conversion
- g(α):
-
integral conversion function
- ΔG:
-
change in free energy, MJ/mol
- ΔH:
-
change in enthalpy, MJ/mol
- k(T):
-
temperature dependent reaction rate constant
- mo :
-
mass of biomass, kg
- mt :
-
mass of biomass at time t, kg
- mf :
-
final mass of biomass, kg
- P(x):
-
conversion function (= x−2e-x)
- R:
-
universal gas constant (= 8.314 J/mol·K)
- t:
-
time, s or min
- T:
-
temperature, K
- α:
-
conversion
- β:
-
heating rate, oC/min
- AC:
-
ash content, %
- CR:
-
Coats–Redfern
- DSC:
-
differential scanning calorimeter
- DTG:
-
differential thermo-gravimetric analysis
- FC:
-
fixed carbon, %
- FTIR:
-
Fourier Transform infrared
- FWO:
-
Flynn Wall Ozawa
- GCMS:
-
Gas chromatograph/mass spectrometer
- HHV:
-
higher heating value, MJ/kg
- KAS:
-
Kissinger-Akahira-Sunose
- KW:
-
kitchen waste/grain waste
- MC :
-
moisture content, %
- TGA:
-
thermo-gravimetric analysis
- VM:
-
volatile matter, %
References
Xue B, Geng Y, Ren WX, Zhang ZL, Zhang WW, Lu CY, Chen XP (2011) An overview of municipal solid waste management in Inner Mongolia Autonomous Region, China. J Mater Cycles Waste Manag 13:283–292. https://doi.org/10.1007/s10163-011-0024-y
Vavouraki AI, Volioti V, Kornaros ME (2014) Optimization of thermo-chemical pretreatment and enzymatic hydrolysis of kitchen wastes. Waste Manag 34:167–173. https://doi.org/10.1016/j.wasman.2013.09.027
Girotto F, Alibardi L, Cossu R (2015) Food waste generation and industrial uses: a review. Waste Management 45:32–41
Giwa AS, Xu H, Chang F, Zhang X, Ali N, Yuan J, Wang K (2019) Pyrolysis coupled anaerobic digestion process for food waste and recalcitrant residues: fundamentals, challenges, and considerations. Energy Sci Eng 7:2250–2264
Mi HK, Han BS, Yuleum S, In TJ, Jung WK (2013) Evaluation of food waste disposal options in terms of global warming and energy recovery: Korea. Int J Energy Environ Eng 4:1–12. https://doi.org/10.1186/2251-6832-4-1
Easterly JL, Burnham M (1996) Overview of biomass and waste fuel resources for power production. Biomass Bioenergy 10:79–92. https://doi.org/10.1016/0961-9534(95)00063-1
Song Y, Hu J, Liu J, Evrendilek F, Buyukada M (2020) CO2-assisted co-pyrolysis of textile dyeing sludge and hyperaccumulator biomass: dynamic and comparative analyses of evolved gases, bio-oils, biochars, and reaction mechanisms. J Hazard Mater 400:123190
Silva-Martínez RD, Sanches-Pereira A, Ortiz W, Goméz MF, Coelho ST (2020) The state-of-the-art of organic waste to energy in Latin America and the Caribbean: challenges and opportunities. Renew Energy 156:509–525. https://doi.org/10.1016/j.renene.2020.04.056
Zaman, C.Z., Pal, K., Yehye, W.A., Sagadevan, S., Shah, S.T., Adebisi, G.A., Marliana, E., Rafique, R.F., Johan, R.B. Pyrolysis: a sustainable way to generate energy from waste https://doi.org/10.5772/intechopen.69036, 2017
International Energy Agency (2006) Annual report, 2006: IEA bioenergy: task 34, pyrolysis of biomass. International Energy Agency, Paris, France
Czajczyn’ska, D., Anguilano, L., Ghazal, H., Krzyzyn´ ska , H. . Reynolds, A. J., Spencer, and Jouhara, H. Potential of pyrolysis processes in the waste management sector, Thermal Science and Engineering Progress 3 (2017) 171–197
Lin CSK, Pfaltzgraff LA, Herrero-Davila L, Mubofu EB, Abderrahim S, Clark JH, Koutinas AA, Kopsahelis N, Stamatelatou K, Dickson F, Thankappan S, Mohamed Z, Brocklesby R, Luque R (2013) Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ Sci 6:426–464. https://doi.org/10.1039/c2ee23440h
Grycová B, Koutník I, Pryszcz A (2016) Pyrolysis process for the treatment of food waste. Bioresour Technol 218:1203–1207. https://doi.org/10.1016/j.biortech.2016.07.064
Girotto F, Alibardi L, Cossu R (2015) Food waste generation and industrial uses: a review. Waste Manag 45:32–41. https://doi.org/10.1016/j.wasman.2015.06.008
Aguiar L, Márquez-Montesinos F, Gonzalo A, Sánchez JL, Arauzo J (2008) Influence of temperature and particle size on the fixed bed pyrolysis of orange peel residues. J Anal Appl Pyrolysis 83:124–130. https://doi.org/10.1016/j.jaap.2008.06.009
Mopoung S (2008) Surface image of charcoal and activated charcoal from banana peel. J Microsc Soc Thail 22:15–19
Miranda R, Bustos-Martinez D, Blanco CS, Villarreal MHG, Cantú MER (2009) Pyrolysis of sweet orange (Citrus sinensis) dry peel. J Anal Appl Pyrolysis 86:245–251. https://doi.org/10.1016/j.jaap.2009.06.001
Liang S, Han Y, Wei L, McDonald AG (2015) Production and characterization of bio-oil and bio-char from pyrolysis of potato peel wastes. Biomass Convers Biorefinery 5:237–246. https://doi.org/10.1007/s13399-014-0130-x
Apaydin-Varol E, Pütün E, Pütün AE (2007) Slow pyrolysis of pistachio shell. Fuel 86:1892–1899. https://doi.org/10.1016/j.fuel.2006.11.041
Haykiri-Acma H (2006) The role of particle size in the non-isothermal pyrolysis of hazelnut shell. J Anal Appl Pyrolysis 75:211–216. https://doi.org/10.1016/j.jaap.2005.06.002
González JF, Román S, Encinar JM, Martínez G (2009) Pyrolysis of various biomass residues and char utilization for the production of activated carbons. J Anal Appl Pyrolysis 85:134–141. https://doi.org/10.1016/j.jaap.2008.11.035
Purevsuren B, Avid B, Gerelmaa T, Davaajav Y, Morgan TJ, Herod AA, Kandiyoti R (2004) The characterisation of tar from the pyrolysis of animal bones. Fuel 83:799–805. https://doi.org/10.1016/j.fuel.2003.10.011
Ayllón M, Aznar M, Sánchez JL, Gea G, Arauzo J (2006) Influence of temperature and heating rate on the fixed bed pyrolysis of meat and bone meal. Chem Eng J 121:85–96. https://doi.org/10.1016/j.cej.2006.04.013
Liu H, Ma X, Li L, Hu Z, Guo P, Jiang Y (2014) The catalytic pyrolysis of food waste by microwave heating. Bioresour Technol 166:45–50. https://doi.org/10.1016/j.biortech.2014.05.020
Zhang B, Zhong Z, Min M, Ding K, Xie Q, Ruan R (2015) Catalytic fast co-pyrolysis of biomass and food waste to produce aromatics: analytical Py-GC/MS study. Bioresour Technol 189:30–35. https://doi.org/10.1016/j.biortech.2015.03.092
Kumar M, Sabbarwal S, Mishra PK, Upadhyay SN (2019b) Thermal degradation kinetics of sugarcane leaves (Saccharum officinarum L) using thermo-gravimetric and differential scanning calorimetric studies. Bioresour Technol 279:262–270. https://doi.org/10.1016/j.biortech.2019.01.137
Sriram A, Swaminathan G (2018) Pyrolysis of Musa balbisiana flower petal using thermogravimetric studies. Bioresour Technol 265:236–246. https://doi.org/10.1016/j.biortech.2018.05.043
Ozawa T (1965) A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn 38:1881–1886
Akahira T, Sunose T (1971) Method of determining activation deterioration constant of electrical insulating materials. Res Report Chiba Inst Technol 16:22–23
Doyle CD (1965) Series approximations to the equation of thermogravimetric data [13]. Nature 207:290–291. https://doi.org/10.1038/207290a0
Starink, M.J., 2017. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochimica Acta 404(1–2):163–176.https://doi.org/10.1016/S0040-6031(03)00144-8
Wanjun T, Cunxin W, Donghua C (2006) An investigation of the pyrolysis kinetics of some aliphatic amino acids. Jounral Anal Appl Pyrolysis 75:49–53. https://doi.org/10.1016/j.jaap.2005.04.003
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201:68–69
Criado JM, Malek J, Ortega A (1989) Applicability of the master plots in kinetic analysis of non-isothermal data. Thermochim Acta 147:377–385
Kumar M, Upadhyay SN, Mishra PK (2020b) Effect of Montmorillonite clay on pyrolysis of paper mill waste. Bioresour Technol 123161:123161. https://doi.org/10.1016/j.biortech.2020.123161
Chen, H., 2018. Co-pyrolysis kinetics and behaviours of kitchen waste and Chlorella vulgaris using thermogravimetric analyzer and fixed bed reactor. Energy Convers. Manag. 165, 45–52. https://doi.org/10.1037/0033-2909.I26.1.78
Li P, Xie Y, Zeng Y, Hu W, Kang Y, Li X, Wang Y, Xie T, Zhang Y (2017) Bioconversion of welan gum from kitchen waste by a two-step enzymatic hydrolysis pretreatment. Appl Biochem Biotechnol 183:820–832. https://doi.org/10.1007/s12010-017-2466-8
Luo S, Xiao B, Hu Z, Liu S (2010) Effect of particle size on pyrolysis of single-component municipal solid waste in fixed bed reactor. Int J Hydrog Energy 35:93–97
Mehmood MA, Ye G, Luo H, Liu C, Malik S, Afzal I, Xu J, Ahmad MS (2017) Pyrolysis and kinetic analyses of Camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour Technol 228:18–24. https://doi.org/10.1016/j.biortech.2016.12.096
Kumar M, Upadhyay SN, Mishra PK (2019c) A comparative study of thermochemical characteristics of lignocellulosic biomasses. Bioresour. Technol. Reports 8:100186. https://doi.org/10.1016/j.biteb.2019.100186
Müsellim E, Tahir MH, Ahmad MS, Ceylan S (2018) Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl Therm Eng 137:54–61. https://doi.org/10.1016/j.applthermaleng.2018.03.050
Collins S, Ghodke P (2018) Kinetic parameter evaluation of groundnut shell pyrolysis through use of thermogravimetric analysis. J Environ Chem Eng 6:4736–4742. https://doi.org/10.1016/j.jece.2018.07.012
Kumar M, Mishra PK, Upadhyay SN (2019a) Pyrolysis of Saccharum munja: optimization of process parameters using response surface methodology (RSM) and evaluation of kinetic parameters. Bioresour Technol Reports 8:100332. https://doi.org/10.1016/j.biteb.2019.100332
Biswas B, Pandey N, Bisht Y, Singh R, Kumar J, Bhaskar T (2017) Pyrolysis of agricultural biomass residues: comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour Technol 237:57–63. https://doi.org/10.1016/j.biortech.2017.02.046
Smidt, E., Schwanninger, M., 2005. Spectroscopy letters: an international journal for rapid characterization of waste materials using FTIR spectroscopy: process monitoring and quality assessment 247–270. https://doi.org/10.1081/SL-200042310
Chatterjee G, Shadangi KP, Mohanty K (2018) Fuel properties and composition study of Cassia siamea seed crude pyrolytic oil and char. Fuel 234:609–615. https://doi.org/10.1016/j.fuel.2018.07.066
Jo HH, Kim SS, Shin JW, Lee YE, Yoo YS (2017) Pyrolysis characteristics and kinetics of food wastes. Energies 10:1191. https://doi.org/10.3390/en10081191
Bach QV, Chen WH (2017) A comprehensive study on pyrolysis kinetics of microalgal biomass. Energy Convers Manag 131:109–116. https://doi.org/10.1016/j.enconman.2016.10.077
Mishra RK, Mohanty K (2018) Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour Technol 251:63–74
Islam MA, Kabir G, Asif M, Hameed BH (2015) Combustion kinetics of hydrochar produced from hydrothermal carbonisation of Karanj (Pongamia pinnata) fruit hulls via thermogravimetric analysis. Bioresour Technol 194:14–20. https://doi.org/10.1016/j.biortech.2015.06.094
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19. https://doi.org/10.1016/j.tca.2011.03.034
El-Sayed SA, Mostafa ME (2014) Pyrolysis characteristics and kinetic parameters determination of biomass fuel powders by differential thermal gravimetric analysis (TGA/DTG). Energy Convers Manag 85:165–172
Ahmad MS, Mehmood MA, Taqvi STH, Elkamel A, Liu CG, Xu J, Rahimuddin SA, Gull M (2017b) Pyrolysis, kinetics analysis, thermodynamics parameters and reaction mechanism of Typha latifolia to evaluate its bioenergy potential. Bioresour.Technol. 245:491–501
Kumar M, Mishra PK, Upadhyay SN (2020a) Thermal degradation of rice husk: effect of pre-treatment on kinetic and thermodynamic parameters. Fuel 268:117164. https://doi.org/10.1016/j.fuel.2020.117164
Asadullah M, Ab Rasid NS, Kadir SASA, Azdarpour A (2013) Production and detailed characterization of bio-oil from fast pyrolysis of palm kernel shell. Biomass Bioenergy 59:316–324. https://doi.org/10.1016/j.biombioe.2013.08.037
Morali U, Şensöz S (2015) Pyrolysis of hornbeam shell (Carpinus betulus L.) in a fixed bed reactor: characterization of bio-oil and bio-char. Fuel 150:672–678. https://doi.org/10.1016/j.fuel.2015.02.095
David E, Kopac J (2018) Pyrolysis of rapeseed oil cake in a fixed bed reactor to produce bio-oil. J Anal Appl Pyrolysis 134:495–502. https://doi.org/10.1016/j.jaap.2018.07.016
Şen N, Kar Y (2011) Pyrolysis of black cumin seed cake in a fixed-bed reactor. Biomass Bioenergy 35:4297–4304. https://doi.org/10.1016/j.biombioe.2011.07.019
Chen D, Yin L, Wang H, He P (2014) Pyrolysis technologies for municipal solid waste: a review. Waste Manag 34:2666–2486. https://doi.org/10.1016/j.wasman.2014.08.004
Aysu T, Abd Rahman NA, Sanna A (2016) Catalytic pyrolysis of Tetraselmis and Isochrysis microalgae by nickel ceria based catalysts for hydrocarbon production. Energy 103:205–214. https://doi.org/10.1016/j.energy.2016.02.055
Acknowledgments
The authors are grateful to the head of the Department of Chemical Engineering & Technology and coordinator of the Sophisticated Laboratory and Central Instrument Facility Centre, Indian Institute of Technology (BHU) Varanasi. The authors would also like to thank the in charge of the Advanced Instrumentation Research Facility (AIRF) JNU, New Delhi, for carrying out the GCMS analysis. One of the authors (MK) is grateful to the MHRD, New Delhi, for the award of a senior research fellowship.
Author information
Authors and Affiliations
Contributions
Mohit Kumar carried out the literature search, conducted all experiments, analyzed the experimental data, and prepared first draft of manuscript.
Neha Srivastava assisted in the conduct of experiments and preparation of the first draft of the manuscript.
S. N. Upadhyay helped in planning the experiments, analysis of data, and finalization of the manuscript and supervised the entire work.
P. K. Mishra helped in planning the experiments, analysis of data, and finalization of the manuscript and supervised the entire work.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, M., Srivastava, N., Upadhyay, S.N. et al. Thermal degradation of dry kitchen waste: kinetics and pyrolysis products. Biomass Conv. Bioref. 13, 2779–2796 (2023). https://doi.org/10.1007/s13399-021-01309-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01309-z