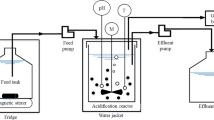
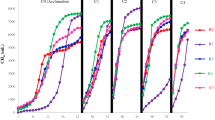
Abstract
This study carried out an anaerobic co-digestion treatment of cheese whey using cattle manure as a co-substrate. A co-digestion process was implemented in three tubular reactors, each one with a different organic load rate (0.5, 1.0 and 1.5 kg CODfed m−3digester day−1). A biochemical and microbiological analysis along the axial axis of each reactor showed that it was possible to obtain a phase separation with an organic load rate of 1.0 kg CODfed m−3digester day−1. The volatile fatty acid production phase takes place in the first reactor section and the consumption phase in the second section. Results from high-throughput sequencing analysis showed differences in the microbial communities between the samples of the three reactors for both eubacterial and archaeal compositions. In the reactor with intermediate organic loading rate, acetoclastic communities predominate, while at lower load, both acetoclastic and hydrogenotrophic communities are detected. Additionally, a microbial consortium stratification was observed.






Similar content being viewed by others
Availability of data and material
The information supporting the conclusions of this article are included within the document and as supplementary data. To reinforce research findings, some data are presented and as an attached spreadsheet file.
Abbreviations
- ACoD:
-
Anaerobic co-digestion
- AD:
-
Anaerobic digestion
- BMP:
-
Biomethane potential
- BPR:
-
Biogas production rate
- C/N:
-
Carbon/nitrogen ratio
- CA:
-
Correspondence analysis
- CM:
-
Cattle manure
- CMS:
-
Cattle manure sludge
- COD:
-
Chemical oxygen demand
- CW:
-
Cheese whey
- DNA:
-
Deoxyribonucleic acid
- OLR:
-
Organic load rate
- OTU:
-
Operational taxonomic unit
- PCR:
-
Polymerase chain reaction
- SBP:
-
Specific biogas production
- SMA:
-
Specific methanogenic activity
- SP:
-
Sampling port
- SPP:
-
Struvite precipitation potential
- TA:
-
Total alkalinity
- TR:
-
Tubular reactor
- VFA:
-
Volatile fatty acid
- VS:
-
Volatile solids
References
Rashama C, Ijoma G, Matambo T (2019) Biogas generation from by-products of edible oil processing: a review of opportunities, challenges and strategies. Biomass Convers Biorefinery 9(4):803–826. https://doi.org/10.1007/s13399-019-00385-6
Elsayed M, Diab A, Soliman M (2020) Methane production from anaerobic co-digestion of sludge with fruit and vegetable wastes : effect of mixing ratio and inoculum type. Biomass Convers Biorefinery:1–10. https://doi.org/10.1007/s13399-020-00785-z
Demirel B, Yenigun O, Onay TT (2005) Anaerobic treatment of dairy wastewaters: a review. Process Biochem 40(8):2583–2595. https://doi.org/10.1016/j.procbio.2004.12.015
Comino E, Riggio VA, Rosso M (2012) Biogas production by anaerobic co-digestion of cattle slurry and cheese whey. Bioresour Technol 114:46–53. https://doi.org/10.1016/j.biortech.2012.02.090
Marshall K, Quiros-Campos C, Van der Werf JHJ, Kinghorn B (2011) Marker-based selection within smallholder production systems in developing countries. Livest Sci 136(1):45–54. https://doi.org/10.1016/j.livsci.2010.09.006
Guinee TP, Carić M, Kalab M (2004) Pasteurized processed cheese and substitute/imitation cheese products. In Cheese: chemistry, physics and microbiology 2:349–394. https://doi.org/10.1016/S1874-558X(04)80052-6
Brandelli A, Daroit DJ, Corrêa APF (2015) Whey as a source of peptides with remarkable biological activities. Food Res Int 73:149–161. https://doi.org/10.1016/j.foodres.2015.01.016
Fox PF, Guinee TP, Cogan TM, McSweeney PLH (2016) Whey and whey products. In: Fundamentals of cheese science, Second. Springer, US, pp 755–768
Escalante H, Castro L, Amaya MP, Jaimes L, Jaimes-Estévez J (2018) Anaerobic digestion of cheese whey: energetic and nutritional potential for the dairy sector in developing countries. Waste Manag 71:711–718. https://doi.org/10.1016/j.wasman.2017.09.026
Escalante-Hernández H, Castro-Molano LDP, Besson V, Jaimes-Estévez J (2017) Feasibility of the anaerobic digestion of cheese whey in a plug flow reactor (PFR) under local conditions. Ing Investig Tecnol 18(3):264–277. https://doi.org/10.22201/fi.25940732e.2017.18n3.024
Fernández C, Cuetos MJ, Martínez EJ, Gómez X (2015) Thermophilic anaerobic digestion of cheese whey: coupling H2 and CH4 production. Biomass Bioenergy 81:55–62. https://doi.org/10.1016/j.biombioe.2015.05.024
Wang K, Yin J, Shen D, Li N (2014) Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: effect of pH. Bioresour Technol 161:395–401. https://doi.org/10.1016/j.biortech.2014.03.088
Venkateshkumar R, Shanmugam S, Veerappan AR (2019) Experimental investigation on the effect of anaerobic co-digestion of cotton seed hull with cow dung. Biomass Convers Biorefinery:1–8. https://doi.org/10.1007/s13399-019-00523-0
Hagos K, Zong J, Li D, Liu C, Lu X (2017) Anaerobic co-digestion process for biogas production: progress, challenges and perspectives. Renew Sust Energ Rev 76(2016):1485–1496. https://doi.org/10.1016/j.rser.2016.11.184
Rico C, Muñoz N, Fernández J, Rico JL (2015) High-load anaerobic co-digestion of cheese whey and liquid fraction of dairy manure in a one-stage UASB process: limits in co-substrates ratio and organic loading rate. Chem Eng J 262:794–802. https://doi.org/10.1016/j.cej.2014.10.050
Castro L et al (2017) Low cost digester monitoring under realistic conditions: rural use of biogas and digestate quality. Bioresour Technol 239(May):311–317. https://doi.org/10.1016/j.biortech.2017.05.035
Kumar P, Samuchiwal S, Malik A (2020) Anaerobic digestion of textile industries wastes for biogas production. Biomass Convers Biorefinery 10:1–10. https://doi.org/10.1007/s13399-020-00601-8
Brown N, Güttler J, Shilton A (2016) Overcoming the challenges of full scale anaerobic co-digestion of casein whey. Renew Energy 96:425–432. https://doi.org/10.1016/j.renene.2016.04.044
Martí-Herrero J, Cipriano J (2012) Design methodology for low cost tubular digesters. Bioresour Technol 108:21–27. https://doi.org/10.1016/j.biortech.2011.12.117
Grant WD, Lawrence TM (2014) A simplified method for the design and sizing of anaerobic digestion systems for smaller farms. Enviro Dev Sustain 16(2):345–360. https://doi.org/10.1007/s10668-013-9480-y
Papurello D, Silvestri S, Tomasi L, Belcari I, Biasioli F, Santarelli M (2016) Biowaste for SOFCs. Energy Procedia 101(September):424–431. https://doi.org/10.1016/j.egypro.2016.11.054
Kinyua MN, Zhang J, Camacho-Céspedes F, Tejada-Martinez A, Ergas SJ (2016) Use of physical and biological process models to understand the performance of tubular anaerobic digesters. Biochem Eng J 107:35–44. https://doi.org/10.1016/j.bej.2015.11.017
Shigehisa I, Takane K (1994) Principles of microbial film process. In: Wastewater treatment with microbial films. Wiley, Hoboken, p 183
Amani T, Nosrati M, Sreekrishnan TR (2010) Anaerobic digestion from the viewpoint of microbiological, chemical, and operational aspects - a review. Environ Rev 18(1):255–278. https://doi.org/10.1139/A10-011
Namsree P, Suvajittanont W, Puttanlek C, Uttapap D, Rungsardthong V (2012) Anaerobic digestion of pineapple pulp and peel in a plug-flow reactor. J Environ Manag 110:40–47. https://doi.org/10.1016/j.jenvman.2012.05.017
Angelidaki I, Karakashev D, Batstone DJ, Plugge CM, Stams AJ (2011) Biomethanation and its potential. Methods Enzymol 494(6):327–351. https://doi.org/10.1016/B978-0-12-385112-3.00016-0
Biderre-Petit C et al (2016) Distribution of Dehalococcoidia in the anaerobic deep water of a remote meromictic crater lake and detection of Dehalococcoidia-derived reductive dehalogenase homologous genes. PLoS One 11(1):e0145558. https://doi.org/10.1371/journal.pone.0145558
Takai K, Horikoshi K (2000) Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microbiol 66(11):5066–5072. https://doi.org/10.1128/AEM.66.11.5066-5072.2000
Sotres A, Tey L, Bonmatí A, Viñas M (2016) Microbial community dynamics in continuous microbial fuel cells fed with synthetic wastewater and pig slurry. Bioelectrochemistry 111:70–82. https://doi.org/10.1016/j.bioelechem.2016.04.007
Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM (2014) Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42(D1):633–642. https://doi.org/10.1093/nar/gkt1244
B. J. Jobling Purser, S. M. Thai, T. Fritz, S. R. Esteves, R. M. Dindale, and A. J. Guwy, ‘An improved titration model reducing over estimation of total volatile fatty acids in anaerobic digestion of energy crop, animal slurry and food waste’, Water Res, vol. 61, no. 0, pp. 162–170, 2014, https://doi.org/10.1016/j.watres.2014.05.020
American Public Health Association (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA): Washington, DC
Astals S, Batstone DJ, Tait S, Jensen PD (2015) Development and validation of a rapid test for anaerobic inhibition and toxicity. Water Res 81:208–215. https://doi.org/10.1016/j.watres.2015.05.063
Mainardis M, Flaibani S, Trigatti M, Goi D (2019) Techno-economic feasibility of anaerobic digestion of cheese whey in small Italian dairies and effect of ultrasound pre-treatment on methane yield. J Environ Manag 246(June):557–563. https://doi.org/10.1016/j.jenvman.2019.06.014
Yang K, Yu Y, Hwang S (2003) Selective optimization in thermophilic acidogenesis of cheese-whey wastewater to acetic and butyric acids: partial acidification and methanation. Water Res 37(10):2467–2477. https://doi.org/10.1016/S0043-1354(03)00006-X
J. Ribera et al., ‘Anaerobic membrane bioreactor (AnMBR) for the treatment of cheese whey for the potential recovery of water and energy’, Waste Biomass Valorization, vol. 0, no. 0, p. 0, 2018, https://doi.org/10.1007/s12649-018-0482-8
Imeni SM, Pelaz L, Corchado-lopo C, Busquets AM, Ponsá S, Colón J (2019) Bioresource technology techno-economic assessment of anaerobic co-digestion of livestock manure and cheese whey ( cow , goat & sheep ) at small to medium dairy farms. Bioresour Technol 291(June):121872. https://doi.org/10.1016/j.biortech.2019.121872
Angelidaki I, Boe K, Ellegaard L (2005) Effect of operating conditions and reactor configuration on efficiency of full-scale biogas plants. Water Sci Technol 52(1–2):189–194. https://doi.org/10.2166/wst.2005.0516
McMahon KD, Stroot PG, Mackie RI, Raskin L (2001) Anaerobic codigestion of municipal solid waste and biosolids under various mixing conditions-II: microbial population dynamics. Water Res 35(7):1817–1827. https://doi.org/10.1016/S0043-1354(00)00438-3
Martí-Herrero J, Ceron M, Garcia R, Pracejus L, Alvarez R, Cipriano X (2015) The influence of users’ behavior on biogas production from low cost tubular digesters: a technical and socio-cultural field analysis. Energy Sustain Dev 27:73–83. https://doi.org/10.1016/j.esd.2015.05.003
Liu Y, Wachemo AC, Yuan HR, Li XJ (2019) Anaerobic digestion performance and microbial community structure of corn stover in three-stage continuously stirred tank reactors. Bioresour Technol 287:121339. https://doi.org/10.1016/j.biortech.2019.121339
Fonseca JP, Hoffmann L, Cabral BCA, Dias VHG, Miranda MR, de Azevedo Martins AC, Boschiero C, Bastos WR, Silva R (2018) Contrasting the microbiomes from forest rhizosphere and deeper bulk soil from an Amazon rainforest reserve. Gene 642:389–397. https://doi.org/10.1016/j.gene.2017.11.039
Zheng L, Zhang Z, Tian L, Zhang L, Cheng S, Li Z, Cang D (2019) Mechanistic investigation of toxicological change in ZnO and TiO2 multi-nanomaterial systems during anaerobic digestion and the microorganism response. Biochem Eng J 147:62–71. https://doi.org/10.1016/j.bej.2019.03.017
Jiang Y, McAdam E, Zhang Y, Heaven S, Banks C, Longhurst P (2019) Ammonia inhibition and toxicity in anaerobic digestion: a critical review. J Water Process Eng 32:100899. https://doi.org/10.1016/j.jwpe.2019.100899
Kouzuma A, Kato S, Watanabe K (2015) Microbial interspecies interactions: recent findings in syntrophic consortia. Front Microbiol 6(MAY). https://doi.org/10.3389/fmicb.2015.00477
Calabrò PS, Fazzino F, Folino A, Scibetta S, Sidari R (2019) Improvement of semi-continuous anaerobic digestion of pre-treated orange peel waste by the combined use of zero valent iron and granular activated carbon. Biomass Bioenergy 129:105337. https://doi.org/10.1016/j.biombioe.2019.105337
L. Treu et al., ‘Microbial profiling during anaerobic digestion of cheese whey in reactors operated at different conditions’, Bioresour Technol, vol. 275, no. October 2018, pp. 375–385, 2019, https://doi.org/10.1016/j.biortech.2018.12.084
Li Y, Wang Y, Yu Z, Lu J, Li D, Wang G, Li Y, Wu Y, Li S, Xu F, Li G, Gong X (2018) Effect of inoculum and substrate/inoculum ratio on the performance and methanogenic archaeal community structure in solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover. Waste Manag 81:117–127. https://doi.org/10.1016/j.wasman.2018.09.042
Funding
The authors gratefully acknowledge funding from the Universidad Industrial de Santander and the Universidad de León for partially supporting the development of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Code availability
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jaimes-Estévez, J., Castro, L., Escalante, H. et al. Cheese whey co-digestion treatment in a tubular system: microbiological behaviour along the axial axis. Biomass Conv. Bioref. 12, 5719–5728 (2022). https://doi.org/10.1007/s13399-020-00988-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00988-4