Abstract
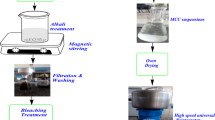
This study reports preparation of biosilica from teff straw ash via sol-gel method. Teff straw ash prepared at different ashing temperatures (500, 700, and 900 °C) was treated with 2.5 N NaOH solution prior to acidification in order to extract biosilica in the form of sodium silicate solution (SSS). The effects of ashing temperatures on biosilica yield and physiochemical properties were studied. Physicochemical properties of extracted biosilica were determined by performing XRD, FE-SEM, EDX, and FTIR analysis. The carbon content and other impurities in extracted biosilica decreased with rise in ashing temperature. The biosilica yield increased with ashing temperature in the order 500 °C (49.5%) < 700 °C (70.2%) < 900 °C (84.5%). EDX, XRF, and FTIR analysis confirmed that the purity was > 99% for biosilica prepared from teff straw ash prepared at 900 °C.





Similar content being viewed by others
References
Adam F, Chew TS, Andas J (2011) A simple template-free sol-gel synthesis of spherical nanosilica from agricultural biomass. J Sol-Gel Sci Technol 59:580–583
Bultosa G (2007) Physicochemical characteristics of grain and flour in 13 tef [Eragrostis tef (Zucc.) Trotter] grain varieties. J Appl Sci Res 3:2042–2050
Kassie BT, Hengsdijk H, Rötter R, Kahiluoto H, Asseng S, Van Ittersum M (2013) Adapting to climate variability and change: experiences from cereal-based farming in the central rift and kobo valleys, Ethiopia. Environ Manag 52:1115–1131
Chaka A, Kenea T, Gebresenbet G (2016) Analysis of the supply chain and logistics practices of warqe food products in Ethiopia. Int J Food System Dynam 7:213–228
Chufo A, Yuan H, Zou D, Pang Y, Li X (2015) Biomethane production and physicochemical characterization of anaerobically digested teff (Eragrostis tef) straw pretreated by sodium hydroxide. Bioresour Technol 181:214–219
Sanchis E, Ferrer M, Calvet S, Coscollà C, Yusà V, Cambra-López M (2014) Gaseous and particulate emission profiles during controlled rice straw burning. Atmos Environ 98:25–31
Wassie AB, Srivastava VC (2016) Teff straw characterization and utilization for chromium removal from wastewater: kinetics, isotherm and thermodynamic modelling. J Environ Chem Eng 4:1117–1125
Wassie AB, Srivastava VC (2016) Chemical treatment of teff straw by sodium hydroxide, phosphoric acid and zinc chloride: adsorptive removal of chromium. Int J Environ Sci Technol 13(10):2415–2426
Abou Rida M, Harb F (2014) Synthesis and characterization of amorphous silica nanoparitcles from aqueous silicates uisng cationic surfactants. J Met Mater Miner 24:37–42
Kalapathy U, Proctor A, Shultz J (2000) A simple method for production of pure silica from rice hull ash. Bioresour Technol 73:257–262
Kalapathy U, Proctor A, Shultz J (2002) An improved method for production of silica from rice hull ash. Bioresour Technol 85:285–289
Athinarayanan J, Periasamy VS, Alhazmi M, Alatiah KA, Alshatwi AA (2014) Synthesis of biogenic silica nanoparticles from rice husks for biomedical applications. Ceram Int 41:275–281
Nazriati N, Setyawan H, Affandi S, Yuwana M, Winardi S (2014) Using bagasse ash as a silica source when preparing silica aerogels via ambient pressure drying. J Non-Cryst Solids 400:6–11
Wassie AB, Srivastava VC (2017) Synthesis and characterization of nano-silica from teff straw. J Nanopart Res 46:64–72
Bageru AB, Srivastava VC (2017) Preparation and characterisation of biosilica from teff (Eragrostis tef) straw by thermal method. Mater Lett 206:13–17
Hariharan V, Sivakumar G (2013) Studies on synthesized nanosilica obtained from bagasse ash. Int J ChemTech Res 5:1263–1266
Sivakumar G, Amutha K (2012) Studies on silica obtained from cow dung ash. Adv Mater Res 584:470–473
Kow K-W, Yusoff R, Aziz ARA, Abdullah EC (2016) Determination of kinetic parameters for thermal decomposition of bamboo leaf to extract bio-silica. Energy Sources A: Recover Util Environ Eff 38:3249–3254
Nandiyanto ABD, Rahman T, Fadhlulloh MA, Abdullah AG, Hamidah I, Mulyanti B (2016) Synthesis of silica particles from rice straw waste using a simple extraction method. IOP Conf Ser Mater Sci Eng 128:12040
Rahman IA, Vejayakumaran P, Sipaut CS, Ismail J, Abu Bakar M, Adnan R, Chee CK (2006) Effect of anion electrolytes on the formation of silica nanoparticles via the sol-gel process. Ceram Int 32:691–699
Chakraverty A, Kaleemullah S (1991) Conversion of rice husk into amorphous silica and combustible gas. Energy Convers Manag 32:565–570
Li X, Cao Z, Zhang Z, Dang H (2006) Surface-modification in situ of nano-SiO2 and its structure and tribological properties. Appl Surf Sci 252:7856–7861
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40:997–1026
Alvarez E, Blanco J, Avila P, Knapp C (1999) Activation of monolithic catalysts based on diatomaceous earth for sulfur dioxide oxidation. Catal Today 53:557–563
Ribeiro PC, Kiminami RHGA, Costa ACFM (2014) Nanosilica synthesized by the Pechini method for potential application as a catalytic support. Ceram Int 40 (2035–2039
Awizar DA, Othman NK, Jalar A, Daud AR, Rahman IA, Al-Hardan NH (2013) Nanosilicate extraction from rice husk ash as green corrosion inhibitor. Int J Electrochem Sci 8:1759–1769
Barker AV, Pilbeam DJ (2006) Handbook of plant nutrition, Taylor & Francis - CRC Press, ISBN 9780824759049
Oudenhoven SRG, van der Ham AGJ, van den Berg H, Westerhof RJM, Kersten SRA (2016) Using pyrolytic acid leaching as a pretreatment step in a biomass fast pyrolysis plant: process design and economic evaluation. Biomass Bioenergy 95:388–404
Kim KD, Kim HT (2002) Formation of silica nanoparticles by hydrolysis of TEOS using a mixed semi-batch/batch method. J Sol-Gel Sci Technol 25:183–189
Sobrosa FZ, Stochero NP, Marangon E, Tier MD (2017) Development of refractory ceramics from residual silica derived from rice husk ash. Ceram Int 43:7142–7146
Cui Y, Bu X, Zou H, Xu X, Zhou D, Liu H, Zhang X, Liu Y, Sun H, Jiang J, Zhang H (2017) A dual solvent evaporation route for preserving carbon nanoparticle fluorescence in silica gel and producing white light-emitting diodes. Mater Chem Front 1:387–393
Eynde EV, Lenaerts B, Tytgat T, Verbruggen SW, Hauchecorne B, Blustc R, Lenaerts S (2014) Effect of pretreatment and temperature on the properties of Pinnularia biosilica frustules. RSC Adv 4:56200–56206
Li D, Chen D, Zhu X (2011) Reduction in time required for synthesis of high specific surface area silica from pyrolyzed rice husk by precipitation at low pH. Bioresour Technol 102:7001–7003
Sola-Rabada A, Sahare P, Hickman GJ, Vasquez M, Canham LT, Perry CC, Agarwal V (2018) Biogenic porous silica and silicon sourced from Mexican giant horsetail (Equisetum myriochaetum) and their application as supports for enzyme immobilization. Colloids Surfaces B: Bioint 166:195–202
Qi Y, Wang J, Wang X, Cheng JJ, Wen Z (2017) Selective adsorption of Pb(II) from aqueous solution using porous biosilica extracted from marine diatom biomass: properties and mechanism. Appl Surf Sci 396:965–977
Kamath SR, Proctor A (1998) Silica gel from rice husk ash preparation and characterization. Cereal Chem 75:484–487
Lee JH, Kwon JH, Lee JW, Lee HS, Chang JH, Sang BI (2017) Preparation of high purity silica originated from rice husks by chemically removing metallic impurities. J Ind Eng Chem 50:79–85
Alves RH, da Silva Reis TV, Rovani S, Fungaro DA (2017) Green synthesis and characterization of biosilica produced from sugarcane waste ash. J Chemother 2017(6129035):1–9
Bageru AB, Srivastava VC (2018) Efficient teff-straw based biocomposites with chitosan and alginate for pyridine removal. Int J Environ Sci Technol https://doi.org/10.1007/s13762-018-1957-7
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bageru, A.B., Srivastava, V.C. Biosilica preparation from abundantly available African biomass Teff (Eragrostis tef) straw ash by sol-gel method and its characterization. Biomass Conv. Bioref. 8, 971–978 (2018). https://doi.org/10.1007/s13399-018-0335-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-018-0335-5