Abstract
This study comprehensively evaluates the radiation attenuation efficiencies of hematite and barite, commonly used materials in radiation shielding, using theoretical and simulation investigations. The MCNP-5 code was used to obtain the linear attenuation coefficient (LAC) within the energy range of 0.015–15 MeV, with validation by the XCOM program. Based on these LAC values, various gamma-ray shielding parameters were determined: mass attenuation coefficient, half-value layer, radiation protection capacity, mean free path, transmission factor, and equivalent thickness to lead (ETPb). Additionally, effective atomic number (Zeff) and electron density (Neff) were calculated, including both single-energy and energy-dependent forms for photon absorption and interaction. Furthermore, MCNP-5 simulations and NGCal program calculations were used to assess thermal neutron attenuation, while the NXcom program determined fast neutron behavior. This analysis revealed superior γ-ray shielding for barite compared to hematite. Similarly, the NXcom program indicated better fast neutron shielding for barite. However, interestingly, simulations validated a 210% higher effectiveness in thermal neutron attenuation for hematite. Finally, comparing the studied materials with other shielding materials demonstrated promising potential as environmentally friendly alternatives for effective shielding against various radiation types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nuclear energy plays a significant role as a low-carbon energy source, providing a reliable main source of power in many countries. However, safety and waste disposal remain ongoing challenges [1]. The uncontrolled release of radiation from this technology poses a significant public health concern. As is known, the danger of these radiations lies in their ability to trigger developmental irregularities, leading to birth defects, genetic damage, malignancies, and declining fertility and fitness [2]. Therefore, nuclear scientists have developed many materials as a shield against these radiations, including concrete, mortars, glasses, and polymers [3,4,5,6,7,8,9]. Additionally, there is recent research about using natural raw materials as a radiation shield. Minerals are one of these natural raw materials such as halloysite [10, 11], barite [12, 13], chambersite [14], magnetite, limonite, hematite [13], garnet [15], serpentine [16, 17], and quartz [18]. Hence, the importance of applied mineralogy, as a branch of geology, appears in some recent studies to characterize the application of different minerals as barriers to attenuate the radiation or restrict the radioactive wastes generated from nuclear facilities [19]. According to global initiatives for sustainability, the use of minerals and rocks, in their native status, in radiation attenuation is preferred over concrete, which remains the most predominant material to mitigate the effects of radiation leaks [20,21,22,23,24]. This preference can be attributed to several factors: lower cement usage (compared to the concrete industry) resulting in energy and cost savings, as well as reduced maintenance requirements. Additionally, it allows for the utilization of abundant mineral resources that might not have other uses, effectively repurposing them for nuclear waste storage. Notably, hematite and barite are the most commonly used minerals for γ-ray shielding due to their high density [25,26,27]. They also show efficiency in fast neutron shielding through a moderation process involving inelastic collisions of fast neutrons with barium and iron in barite and hematite, respectively [27, 28]. Employing 137Cs and 60Co at 0.662 and 1.33 MeV, respectively, Akkurt et al. studied the γ-ray attenuation of barite and found good agreement between their experimental findings and the XCOM calculations [12]. Furthermore, various γ-ray shielding parameters have been measured for barite and hematite at different photon energies produced from 133Ba and 152Eu using HPGe detector [13]. The results confirmed that both barite and hematite minerals can efficiently shield γ-rays, with barite exhibiting superior performance. However, few studies, have discussed the radiation attenuation capacity of hematite and barite in their native status [12, 13]. Moreover, these reports, lack data on the fast neutron attenuation of these minerals. Additionally, no theoretical or simulation studies have yet confirmed the experimental findings.
In the Western Desert of Egypt, specifically in the Bahariya Oasis, hydrothermal solutions during the Middle Eocene led to the formation of barite and hematite [29]. Baharia Oasis holds significant, yet unexploited, reserves of these minerals, with hematite alone estimated at around 270 million metric tons [30]. As a result, the hematite served as the main raw material for steel manufacturing at the Egyptian Iron and Steel Company (EISC). However, mining operations for hematite ceased after the government's decision to liquidate EISC in January 2021 due to the high expenses associated with its maintenance and operation, leading to the abandonment of millions of tons of ore. Accordingly, the hematite and barite minerals became like mining wastes with no utilization. Recently, there has been a general approach in the scientific community toward exploiting different types of waste to achieve sustainable development [31,32,33,34,35,36,37,38,39]. Also, this abundance suggests their potential as stable underground repositories for nuclear waste or as wall-lining tiles in radiotherapy and nuclear reactor facilities.
On the other hand, theoretical and simulation software, such as Phy-X/PSD, NXcom, XCOM, WinXCom, and Monte Carlo simulations, have been successfully verified by experimental investigations of radiation attenuation in various materials [40,41,42,43,44,45,46,47,48,49,50,51]. Additionally, some researchers have relied solely on this software to characterize the radiation-shielding properties of other materials [52,53,54,55,56,57,58,59,60,61,62,63,64]. Therefore, this study aims to comprehensively evaluate the gamma-ray, thermal neutron, and fast neutron shielding capabilities of hematite and barite from the Bahariya Oasis. We will achieve this through theoretical and simulation studies of thermal neutrons, fast neutrons, and gamma-ray attenuation. Specifically, the NGCal and NXcom programs will be used for theoretical calculations of thermal and fast neutrons, respectively. Meanwhile, the MCNP-5 code (Monte Carlo simulation code) will be employed for gamma-ray and thermal neutron simulation studies, with validation provided by the XCOM program. Finally, the radiation attenuation of these minerals will be compared to those of commonly used radiation shielding materials.
2 Materials and Methods
2.1 Material Characterization
Hematite and barite samples were derived from Bahariya oasis, the Western Desert in Egypt. The collected sample of barite was characterized by its pale brown-yellow color, glassy luster, and high specific gravity. In contrast, hematite displayed various shades of red, such as dark red, along with an earthy texture and a lower specific gravity than barite. In addition to its earthy luster, a distinguishing feature of the hematite sample was its tendency to leave a residue of its color on the hand. The density and elemental composition of both samples were determined to serve as input data for theoretical and simulation programs used to characterize their radiation attenuation capabilities. As described in [65], the density was characterized. Elemental analysis was performed using X-ray fluorescence (XRF) based on ASTM D7348 and E1621 [66, 67].
2.2 γ-Ray Attenuation (MCNP-5 and XCOM)
2.2.1 MCNP-5 Simulation
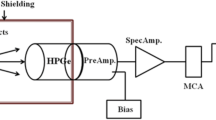
The Monte Carlo simulation code (MCNP-5) is a valuable means to simulate the attenuation parameters of different attenuating materials like concretes [68], glasses [69, 70], polymers, and building materials. MCNP-5 simulation was accomplished via an input file describing material densities, chemical composition, radiation source location, working energies, geometry, and detector. These aforementioned factors were introduced to the input file through many cards like surface, cell, material, source (SDEF), importance, tally, cutoff, and physical. The nuclear library ENDF/B-VI.8 was attached to the MCNP-5 code to support it by the cross-section of interactions required to evaluate the shielding parameters. In the created input file, F4 tally was applied to estimate the average flux per unit cell and the average track length (ATL) that is required to evaluate the linear attenuation coefficient (LAC, cm−1) of the investigated materials. Additionally, the input file contains a cell card that describes each simulation factor (i.e., outer shielding material, collimators, detector, source, and the investigated materials) by a unit cell. Every cell has its density, importance, and cell number. To identify every cell, it should be surrounded by some surfaces which are introduced in the surface card, where every surface has a definite shape and dimensions. For example, the outer shielding material which protects the geometry from the surrounding radiations has a cylindrical shape with a diameter of 25 cm, thickness of 5 cm, and height of 40 cm made of lead. Inside the outer lead cylinder, collimators, samples, and the detector were installed, as illustrated in the 3D representation for the MCNP-5’s input file (Fig. 1). All information about the sources used was introduced to the SDEF card, where the source placed in the center of the outer cylinder POS (0 0 0) emits radiation along Z direction AXS (0 0 1) with energies (ENG) varied between 0.015 and 15 MeV including 0.662 MeV (for Cs-137), 1 MeV, and 1.332 MeV (for Co-60). Additionally, the distribution and emission probability for the radioactive source were introduced to the SDEF card. According to the input file, two lead collimators with narrow apertures were used to collect the γ-ray flux released from the source and absorb the dispersed radiation from both the source and the sample. Between the γ-ray source and investigated samples, the first collimator (7 cm × 7 cm) with a vertical central aperture with a diameter of 1 cm is located. At a distance of 2 cm from the collimator’s upper surface, the source was located inside a hole in the first collimator. Furthermore, the second collimator (7 cm × 3 cm) with a central vertical slit of 1 cm diameter was placed between the sample and the detector. The cutoff card was set at 108 historical emissions. After running the simulation on, an output file is created automatically containing the ATL of γ-photons over the various cells and the relative error in the simulation process which ranges between ± 0.5%. Then, the ATL was used to calculate the LAC for the investigated samples. After that, based on the LAC and the density of the investigated samples, the mass attenuation coefficient (MAC, cm2/g) was evaluated. Additionally, the half-value layer (HVL, cm), transmission factor (TF, %), radiation protection capacity (RPC, %), and equivalent thickness of lead (ETPb, cm) were evaluated based on the simulated LAC according to equations in Table 1.
2.2.2 XCOM
In addition to the MCNP-5 simulation, the XCOM program was used to verify the simulated μ values. Utilizing the NIST database within XCOM, we calculated the MAC values according to the “Mixture Rule” using the following equation[71]:
where \({w}_{i}\) and \(({\mu }_{m}{)}_{i}\) denote the fractional weight and mass attenuation coefficient per element, respectively. Furthermore, Table 2 summarizes different relationships for calculating mean atomic mass, number (<A>, <Z>)mean electron density (<N>), and effective atomic number (Zeff). Zeff takes into account both single and energy-dependent values for photon interaction (PI) and photon energy absorption (PEA). The single Zeff value was determined based on various parameters such as elemental weight fraction [72], fractional electronic content [73,74,75,76], or atomic percentage [77, 78].
2.3 Thermal Neutrons (NGCal Software)
The NGCal software (http://ngcal.com [83]), a free online tool developed by Gökçe et al., calculates the attenuation behavior of thermal neutrons based on the material’s macroscopic cross-section (Σth in cm−1). Σth represents the probability of a neutron being absorbed per unit path length. This software can assess the attenuation performance of materials against thermal (25.4 meV) and fast (4 MeV) neutrons, as well as gamma rays (0.002–20 MeV). Material composition and density are required for calculations.
2.4 Fast Neutrons (NXcom Program)
The macroscopic effective removal cross-section (ΣR) describes the probability of a fast neutron undergoing its first collision within a material, effectively removing it from the group of penetrating neutrons per unit length. The NXcom computer program [84] assesses this removal process for fast neutrons and the attenuation coefficient for gamma rays in various materials (concrete, composites, etc.). Similar to the XCOM program, NXcom requires the elemental composition of a sample as input data and applies the mixture rule to calculate ΣR for each sample as follows[85].
where \({\rho }_{i}\) represents the density of the ith constituent element as in the mixture (\({\rho }_{i}\hspace{0.17em}=\hspace{0.17em}\rho \cdot {w}_{i}\); \(\rho \) represents mixture density), and \({w}_{i}\) denotes the fractionated weight of the ith constituent element.
3 Results and Discussion
3.1 Material Characterization
Table 3 summarizes the elemental composition and density of materials investigated for their radiation shielding effectiveness using simulations and theoretical calculations. The results reveal that barite (4.20 g/cm3) boasts a higher density compared to hematite (2.90 g/cm3). The elemental analysis of barite indicates a primary composition of barium sulfate (BaSO₄), explaining its high concentrations of sulfur (11.83%) and barium (51.93%). Hematite, on the other hand, is composed of Fe₂O₃, leading to its high iron content (61.06%). Interestingly, both minerals contain a significant amount of oxygen, with hematite having a slightly higher proportion.
3.2 γ-Ray Attenuation
3.2.1 Mass Attenuation Coefficient (MAC, cm.2/g)
MAC is the most crucial parameter for radiation shielding, as other parameters are derived from it. Figure 2a–c demonstrates excellent agreement between the simulated MAC values obtained using the MCNP-5 software and those calculated using XCOM, across the energy range of 0.015–15 MeV. This consistency is further supported by the perfect correlations (R2 = 1) observed in Fig. 2a, b for both hematite and barite. The high degree of similarity between the MCNP-5 simulations and XCOM calculations suggests that the simulation process yields highly accurate results. Figure 2c illustrates the simulation findings. The maximum mass attenuation coefficients for hematite and barite occur at the lowest energy level (E = 0.015 MeV), with values of 36.63 cm2/g and 38.35 cm2/g, respectively. As photon energy increases, the MAC values steadily decrease, mainly due to the prevalence of photoelectric absorption. A significant peak (7.38 cm2/g) appears in the MAC of barite at 0.05 MeV, which is attributed to the presence of Ba in its composition. Both materials exhibit similar MAC within the 0.662–3.00 MeV energy range, indicating comparable attenuation behavior. However, pair production becomes the dominant interaction process at higher energies (E > 3 MeV), leading to some variation in the MAC with increasing gamma-ray energy.
3.2.2 Linear Attenuation Coefficient, LAC, (µ, cm.−1)
Figure 3 illustrates the decreasing trend of linear attenuation coefficient (μ) with increasing photon energy (0.015–15 MeV) for hematite and barite. This trend is due to the weakening influence of the photoelectric effect at higher energies. However, a distinct barium K-edge peak disrupts this trend for barite at 0.05 MeV. In the mid-energy range (0.3–3 MeV), Compton scattering becomes the dominant interaction process, leading to a gradual decline in μ. Beyond 5 MeV, pair production takes over as the primary process, resulting in minimal changes in μ for both materials [86]. Notably, barite exhibits consistently higher μ values than hematite.
3.2.3 Half-Value Layer (HVL, cm)
Figure 4 depicts the relationship between photon energy and HVL for hematite and barite. As expected, HVL increases with energy for both materials due to the diminishing influence of photoelectric absorption. A sharp dip in barite’s HVL at 0.05 MeV corresponds to the Barium K-edge. In the intermediate range (0.1–5 MeV), Compton scattering prevails, leading to a gradual HVL rise. Beyond 5 MeV, pair production stabilizes HVL for both materials. Significantly, barite consistently displays lower HVL values than hematite throughout the entire energy range, indicating its superior performance in gamma-ray attenuation. For instance, between 0.015 and 15 MeV, hematite’s HVL values range from 0.01 to 9 cm, while barite's range from 0.004 to 5.20 cm. This suggests that barite consistently demands less thickness to achieve equivalent shielding compared to hematite.
3.2.4 Mean Free Path (MFP, cm)
Figure 5 compares the mean free path (MFP) of hematite and barite with photon energy, mirroring the trend observed for HVL. Notably, barite exhibits consistently lower MFP values throughout the entire energy range (0.015–15 MeV). Barite’s MFP ranges from 0.01 to 7.01 cm, while hematite’s spans from 0.01 to 12.98 cm. A significant increase in MFP for both materials occurs between 0.1 and 5 MeV due to the dominance of Compton scattering. At energies ˃ 5 MeV, the influence of pair production leads to a decrease in the rate of change of MFP.
3.2.5 Transmission Factor (TF, %)
Figure 6 illustrates the dependence of photon transmission factor (TF%) on photon energy. Both hematite and barite exhibit increasing TF% with energy. The lowest TF% (highest attenuation) for both materials occurs at low energies, indicating efficient absorption due to the photoelectric effect. As the energy rises (up to 5 MeV), TF% increases, suggesting a dominance of Compton scattering and a higher probability of photon escape or scattering [86]. At the highest energies (8–15 MeV), both materials show the highest TF% (lowest attenuation), signifying reduced effectiveness against high-energy photons. Notably, barite consistently displays lower TF% values than hematite, demonstrating its superior ability to attenuate photons across the entire energy spectrum.
3.2.6 Radiation protection capacity (RPC, %)
Figure 7 (MCNP-5 simulations) compares the radiation protection capabilities (RPC%) of hematite and barite across different photon energies. While both materials exhibit a decrease in RPC% with increasing energy, hematite generally offers lower protection compared to barite. The only exception is the very low energy range (0.015–0.030 MeV) where both achieve 100% RPC. Barite demonstrates superior performance with higher and more stable RPC% values (100–99.42%) between 0.015–0.100 MeV, while hematite’s peak RPC% (100%) is within a narrower range (0.015–0.050 MeV). The observed differences in RPC% can be explained by the varying influence of photoelectric interaction and Compton scattering on different energy photons. Beyond 0.100 MeV, both materials show a decrease in RPC% due to Compton scattering. At even higher energies (> 5 MeV), the impact of pair production leads to minimal changes in RPC values[86].
3.2.7 Equivalent Thickness to Lead (ETPb, cm)
The thickness of hematite and barite needed to equal 1 cm of lead shielding (ETPb) was calculated for photon energies (0.015–15 MeV) as shown in Fig. 8. In this energy region, hematite required 4.08–70.36 cm, while barite needed 2.92–13.88 cm. The higher µ value of Pb compared to hematite and barite leads to the elevated ETPb values. At the lowest energy (0.015 MeV), ETPb for hematite and barite was 11.95 and 7.89 cm, respectively. ETPb increased at 0.03 MeV and 0.1 MeV due to lead’s L-edge and K-edge effects, which require more hematite or barite to achieve the same level of shielding as lead [81. Between 0.3–1.332 MeV, ETPb decreased to 14.33–6.66 cm for hematite and 4.10–2.95 cm for barite. For energy levels from 3–15 MeV, ETPb increased for hematite (4.57–8.33 cm) and barite (3.13–4.5 cm) due to pair production cross-section correlated with Z2. The highest ETPb for barite (13.88 cm) was observed at 0.03 MeV, while hematite reached its maximum value of 70.36 cm at 0.10 MeV. These results signify the superior shielding capabilities of barite over hematite.
3.2.8 Effective Atomic Number (Zeff), Electron Density (Neff), and Their Derivatives
Table 4 summarizes the calculated parameters for barite and hematite. Notably, barite exhibits higher density, mean atomic number (<Z>), and effective atomic number (Zeff). Based on these preliminary calculations, barite suggests superior efficiency in shielding gamma rays compared to hematite.
Although a single Zeff value has its uses, it is overly simplistic for many applications. For these cases, energy-dependent Zeff values are required. Table 5 shows the maximum (Max.) and minimum (Min.) values of these energy-dependent effective atomic numbers and effective electron densities, categorized for photon interaction (PI) and photon energy absorption (PEA). Notably, the values of average atomic number (<Z>) consistently align with the minimum Zeff, PI across all samples, indicating the dominance of the Compton scattering process (Tables 4 and 5).
Figure 9a, b reveal a high degree of similarity in the variation of Zeff, PI and Zeff, PEA with energy. Therefore, we focus on Zeff, PI (Fig. 9a). Notably, barite (Fig. 9a) lacks the intermediate constant Zeff, PI region. Additionally, a dip appears above 1 MeV, but the minimum remains above <Z>, indicating Compton scattering is not dominant [80]. Finally, Zeff, PI in barite systematically increases more than hematite at higher energies, confirming its superior shielding.
3.3 Thermal Neutron Attenuation
Σth results obtained from the NGCal online software for the investigated samples are confirmed by the MCNP-5 simulation code, as shown in Table 6. The computations illustrate that the thermal neutron attenuation of hematite (0.894 cm⁻1) is higher than that of barite (0.288 cm⁻1) by 210%. The NGCal result matches the MCNP-5 simulation results within about ± 10% difference. The superiority of hematite can be attributed to its higher ratio of iron with a larger absorption cross-Sect. (2.56 barns) compared to barium, in barite, which has a lower absorption cross-Sect. (1.30 barns) [87]. Additionally, hematite has a higher hydrogen (H) content (0.60%) compared to barite (0.13%). Hydrogen has a relatively high absorption cross-section of 0.3326 barns.
3.4 Fast Neutron Attenuation
Table 7 illustrates the weight percentage (wt.%) of each element in the samples and their corresponding removal cross-sections for fast neutrons (ΣR). Oxygen significantly contributes to the ΣR of both barite and hematite, with a higher overall ΣR for barite. For hematite, hydrogen and iron are the major contributors to ΣR, while barium and sulfur play the most significant role in barite. This higher total ΣR value for barite’s constituent elements indicates its superior performance in attenuating fast neutrons compared to hematite.
3.5 Radiation Attenuation Comparison
Table 8 compares the gamma-ray attenuation of the studied samples (hematite and barite) with previously studied materials (concrete, minerals, etc.) using theoretical or simulation methods. Table 8 lists the samples, their descriptions, and gamma-ray linear attenuation coefficients at common comparison energies (0.662, 1.173, and 1.332 MeV). Also, Table 8 illustrates that the barite offers the highest gamma-ray shielding at all three energies. Hematite exhibits superior gamma-ray attenuation to most materials except BRC, BC, WM, H7, HBC7, PVC − C30, and N0 while performing similarly to Nd4.
4 Conclusion
The present study’s findings lead to the following conclusions:
-
1)
Both theoretical calculations (XCOM) and simulations (MCNP-5) demonstrated superior gamma-ray shielding performance for barite compared to hematite. This was evident across all parameters (μ, MAC, HVL, MFP, TF, RPC, ETPb, Zeff, and Neff). The good agreement between theory and simulation supports this conclusion. This advantage can be attributed to barite's higher density (4.20 g/cm3) compared to hematite (2.90 g/cm3).
-
2)
Hematite displayed a 210% higher effectiveness in attenuating thermal neutrons compared to barite, as verified by the MCNP-5 simulation results. This can be explained by the higher absorption cross-section of Fe in hematite (2.56 barns) compared to Ba in barite (1.30 barns). Additionally, hematite has a higher hydrogen content (0.60%) than barite (0.13%).
-
3)
Calculations using NXcom indicated that barite outperforms hematite in shielding fast neutrons. This is due to barite's higher density and barium content, which enhance the probability of inelastic fast neutron collisions.
-
4)
Based on their performance, both hematite and barite have the potential to be used as natural radiation shielding materials, offering an alternative to common shielding materials.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Lee, S.; Kim, M.; Lee, J.: Analyzing the impact of nuclear power on CO2 emissions. Sustainability 9(8), 1428 (2017)
Ryan, J.L.: Ionizing radiation: the good, the bad, and the ugly. J. Investig. Dermatol. 132, 985–993 (2012)
El-Samrah, M.G.; Abdel-Rahman, M.A.E.; El Shazly, R.M.: Effect of heating on physical, mechanical, and nuclear radiation shielding properties of modified concrete mixes. Radiat. Phys. Chem. 153, 104–110 (2018)
Tekin, H.O.; Sayyed, M.I.; Issa, S.A.M.: Gamma radiation shielding properties of the hematite-serpentine concrete blended with WO3 and Bi2O3 micro and nano particles using MCNPX code. Radiat. Phys. Chem. 150, 95–100 (2018)
Azreen, N.M., et al.: Simulation of ultra-high-performance concrete mixed with hematite and barite aggregates using Monte Carlo for dry cask storage. Constr. Build. Mater. 263, 120161 (2020)
Masoud, M.A., et al.: Insights into the effect of the mineralogical composition of serpentine aggregates on the radiation attenuation properties of their concretes. Constr. Build. Mater. 263, 120141 (2020)
Marzuki, A., et al.: The effect of Bi2O3/PbO substitution on physical, optical, structural, and gamma shielding properties of boro-tellurite glasses. Radiat. Phys. Chem. 205, 110722 (2023)
Amin, M., et al.: Improving the brittle behavior of high-strength shielding concrete blended with lead oxide, bismuth oxide, and tungsten oxide nanoparticles against gamma ray. Struct. Eng. Mech. 85(1), 29–53 (2023)
Zayed, A.M., et al.: Physical, mechanical, and radiation attenuation properties of serpentine concrete containing boric acid. Constr. Build. Mater. 272, 121641 (2021)
Mansour, A., et al.: Modified halloysite minerals for radiation shielding purposes. J. Radiat. Res. Appl. Sci. 13(1), 94–101 (2020)
Abu El-Soad, A.M., et al.: Simulation studies for gamma ray shielding properties of Halloysite nanotubes using MCNP-5 code. Appl. Radiat. Isotopes 154, 108882 (2019)
Akkurt, I.; Kilincarslan, S.; Basyigit, C.: The photon attenuation coefficients of barite, marble and limra. Annal. Nucl. Energy. 31(5), 577–582 (2004)
Oto, B., et al.: Investigation of gamma radiation shielding properties of various ores. Prog. Nucl. Energy 85, 391–403 (2015)
Dong, M., et al.: Study of comprehensive shielding behaviors of chambersite deposit for neutron and gamma ray. Prog. Nucl. Energy 146, 104155 (2022)
Kunchariyakun, K.; Sukmak, P.: Utilization of garnet residue in radiation shielding cement mortar. Constr. Build. Mater. 262, 120122 (2020)
Masoud, M.A., et al.: Possibility of using different types of Egyptian serpentine as fine and coarse aggregates for concrete production. Mater. Struct. 53(4), 87 (2020)
Sayyadi, A.; Mohammadi, Y.; Adlparvar, M.R.: Mechanical, Durability, and gamma ray shielding characteristics of heavyweight concrete containing serpentine aggregates and lead waste slag. Adv. Civ. Eng. 2023, 7873637 (2023)
Marquez-Mata, C.A., et al.: Shielding features of seven types on natural quartz. Appl. Radiat. Isotope. 167, 109450 (2021)
Mukherjee, S.: Applied mineralogy: applications in industry and environment. Springer Science and Business Media, Berlin/Heidel-berg Germany (2012)
Hemid, E.M., et al.: Effect of groundwater fluctuation, construction, and retaining system on slope stability of avas hill in Hungary. Open. Geosci. 13(1), 1139–1157 (2021)
Masoud, M.A., et al.: Radiation attenuation assessment of serpentinite rocks from a geological perspective. Toxics 10(11), 697 (2022)
Yılmaz, D.; Kılıç, A.D.: Determination of number, energy and dose albedos of some marbles used in external wall cladding of buildings for 59.54 keV gamma-rays. Radiat. Eff. Defect. Sol. 174(7–8), 668–675 (2019). https://doi.org/10.1080/10420150.2019.1632853
Yılmaz, D.; Kılıc, A.D.; Kalecik, S.: Radiation shielding parameters and Micro-Raman spectral analysis of some pyroclastic rocks. Radiat. Phys. Chem. 203, 110596 (2023)
Büyükyıldız, M.; Kılıç, A.D.; Yılmaz, D.: White and some colored marbles as alternative radiation shielding materials for applications. Radiat. Eff. Defect. Sol. 175(7–8), 657–671 (2020)
Singh, S.; Singh, K.: On the use of green concrete composite as a nuclear radiation shielding material. Prog. Nucl. Energy 136, 103730 (2021)
Lv, Y., et al.: Effect of incorporating hematite on the properties of ultra-high performance concrete including nuclear radiation resistance. Constr. Build. Mater. 327, 126950 (2022)
Zayed, A.M., et al.: Influence of heavyweight aggregates on the physico-mechanical and radiation attenuation properties of serpentine-based concrete. Constr. Build. Mater. 260, 120473 (2020)
Masoud, M.A., et al.: An experimental investigation on the effects of barite/hematite on the radiation shielding properties of serpentine concretes. Prog. Nucl. Energy 120, 103220 (2020)
Baioumy, H.M.: Rare earth elements, S and Sr isotopes and origin of barite from Bahariya Oasis, Egypt: Implication for the origin of host iron ores. J. Afr. Earth Sci. 106, 99–107 (2015)
Said, R.: The Geology of Egypt, 1st edn., p. 734. Routledge, London (1990)
El Maghrabi, A.H., et al.: From hazardous chrysotile and polyamide wastes into sustainable serpentine/polyamide nanocomposite membrane: fabrication, characterization, and environmental application. Sustainability 15(9), 7060 (2023)
Masoud, M.A., et al.: Valorization of hazardous chrysotile by H3BO3 incorporation to produce an innovative eco-friendly radiation shielding concrete: Implications on physico-mechanical, hydration, microstructural, and shielding properties. Cem. Concr. Compos. 141, 105120 (2023)
Zayed, A.M., et al.: From discarded waste to valuable products: barite combination with chrysotile mine waste to produce radiation-shielding concrete. Constr. Build. Mater. 417, 135334 (2024)
Zayed, A.M., et al.: From non-conventional agricultural waste into sustainable and eco-friendly activated carbon through specified thermo-chemical protocol. Appl. Nanosci. 14(1), 21–32 (2024)
Zayed, A.M., et al.: Efficient dye removal from industrial wastewater using sustainable activated carbon and its polyamide nanocomposite derived from agricultural and industrial wastes in column systems. RSC Adv. 13(36), 24887–24898 (2023)
Zayed, A.M., et al.: Facile synthesis of eco-friendly activated carbon from leaves of sugar beet waste as a superior nonconventional adsorbent for anionic and cationic dyes from aqueous solutions. Arab. J. Chem. 16(8), 104900 (2023)
Abdel-Latief, M.A.K., et al.: A new insight upon the use of weathered basalt as alternative raw material in Portland clinker production. Ain Sham. Eng. J. 12(1), 885–896 (2021)
El Shafey, A.M., et al.: Low-cost polyaniline/weathered basalt composites for methylene blue uptake from aqueous solutions. J. Mol. Liq. 390, 123052 (2023)
El-Sheikh, M.N.; Metwally, B.S.; Mubarak, M.F.; Ahmed, H.A.; Moghny, T.A.; Zayed, A.M.: Fabrication of electrospun polyamide–weathered basalt nano-composite as a non-conventional membrane for basic and acid dye removal. Polym. Bull. 80, 8511–8533 (2022)
Şensoy, A.T.; Gökçe, H.S.: Simulation and optimization of gamma-ray linear attenuation coefficients of barite concrete shields. Constr. Build. Mater. 253, 119218 (2020)
Sharifi, S.; Bagheri, R.; Shirmardi, S.P.: Comparison of shielding properties for ordinary, barite, serpentine and steel–magnetite concretes using MCNP-4C code and available experimental results. Ann. Nucl. Energy 53, 529–534 (2013)
Becker, F., et al.: Investigation of various concrete compositions with respect to gamma-radiation transmission properties of Cs-137. Radiat. Phys. Chem. 171, 108679 (2020)
Arslan, H.: Photon attenuation parameters for some tissues from geant4 simulation, theoretical calculations and experimental data: a comparative study. Nucl. Sci. Tech. 30(6), 96 (2019)
Elbashir, B.O., et al.: Comparison of Monte Carlo simulation of gamma ray attenuation coefficients of amino acids with XCOM program and experimental data. Result. Phys. 9, 6–11 (2018)
Verdipoor, K.; Alemi, A.; Mesbahi, A.: Photon mass attenuation coefficients of a silicon resin loaded with WO3, PbO, and Bi2O3 Micro and Nano-particles for radiation shielding. Radiat. Phys. Chem. 147, 85–90 (2018)
Ozel, F., et al.: Production of microstructured BaZrO3 and Ba2P2O7-based polymer shields for protection against ionizing photons. J. Phys. Chem. Sol. 158, 110238 (2021)
Abou-Laila, M.T., et al.: Gamma irradiation effects on styrene butadiene rubber/Pb3O4: Mechanical, thermal, electrical investigations and shielding parameter measurements. Radiat. Phys. Chem. 192, 109897 (2022)
Turşucu, A.; Elmahroug, Y.; Yılmaz, D.: Measurement on radiation shielding parameters of FexCr1_x and FexNix alloys. App. Phys. A 127(8), 640 (2021)
Obaid, S.S., et al.: Attenuation coefficients and exposure buildup factor of some rocks for gamma ray shielding applications. Radiat. Phys. Chem. 148, 86–94 (2018)
Kurtulus, R., et al.: An experimental study and WinXCom calculations on X-ray photon characteristics of Bi2O3- and Sb2O3-added waste soda-lime-silica glass. Ceram. Int. 46(13), 21120–21127 (2020)
Kaçal, M.R., et al.: Evaluation of gamma-ray and neutron attenuation properties of some polymers. Nucl. Eng. Technol. 51(3), 818–824 (2019)
Sayyed, M.I., et al.: Application of the MCNP 5 code to simulate the shielding features of concrete samples with different aggregates. Radiat. Phys. Chem. 174, 108925 (2020)
Sayyed, M.I., et al.: Radiation shielding study of tellurite tungsten glasses with different antimony oxide as transparent shielding materials using MCNPX code. J. Non-Cryst. Sol. 498, 167–172 (2018)
Sayyed, M.I., et al.: Determination of nuclear radiation shielding properties of some tellurite glasses using MCNP5 code. Radiat. Phys. Chem. 150, 1–8 (2018)
Mon, B.Z.; Gili, J.F.; Jecong, M.: Radiation shielding properties of ZnO and other glass modifier oxides: BaO, MgO, Na2O, and TiO2, using EpiXS software. Arab. J. Sci. Eng. 48, 1021–1029 (2022)
Olukotun, S.F., et al.: Neutron-shielding behaviour investigations of some clay-materials. Nucl. Eng. Technol. 51(5), 1444–1450 (2019)
Akkurt, I.; Malidarre, R.B.; Kavas, T.: Monte Carlo simulation of radiation shielding properties of the glass system containing Bi2O3. Eur. Phys. J. Plus. 136(3), 264 (2021)
Al-Obaidi, S., et al.: Neutron shielding calculation for barite-boron-water. Acta Phys. Pol. A. 137, 551–553 (2020)
Yahşi, Ç.Y.: Gamma-ray-shielding parameters of some phantom fabrication materials for medical dosimetry. Emerg. Mater. Res. 10, 307–313 (2021)
Malidarre, R.B.; Kulali, F.; Inal, A.; Oz, A.: Monte Carlo simulation of a waste soda–lime–silica glass system containing Sb2O3 for gamma-ray shielding. Emerg. Mater. Res. 9, 1334–1340 (2020)
Sariyer, D.; Küçer, R.: Effect of different materials to concrete as neutron shielding application. Acta Phys. Pol. A. 137, 477–479 (2020)
Ozan, T.H., et al.: Nuclear radiation shielding competences of barium-reinforced borosilicate glasses. Emerg. Mater. Res. 9, 1131–1144 (2020)
Feride, K.: Simulation studies on the radiological parameters of marble concrete. Emerg. Mater. Res. 9(4), 1341–1347 (2020)
Kaur, U., et al.: Comparative studies of different concretes on the basis of some photon interaction parameters. Appl. Radiat. Isotope. 70(1), 233–240 (2012)
ASTM: ASTM C97, Standard Test Methods for Absorption and Bulk Specific Gravity of Dimension Stone. West Conshohocken, ASTM International (2015)
ASTM: ASTM E1621, Standard guide for elemental analysis by wavelength dispersive X-Ray fluorescence spectrometry. West Conshohocken, ASTM International (2013)
ASTM: ASTM D7348, Standard test methods for Loss on Ignition (LOI) of solid combustion residues. West Conshohocken, ASTM International (2013)
Hassanpour, M., et al.: Studies of the mechanical and neutron shielding features of concrete by incorporation of green additive materials: experimental and numerical study. Radiat. Phys. Chem. 191, 109846 (2022)
Lakshminarayana, G., et al.: B2O3–Bi2O3–TeO2–BaO and TeO2–Bi2O3–BaO glass systems: a comparative assessment of gamma-ray and fast and thermal neutron attenuation aspects. Appl. Phys. A 126(3), 202 (2020)
Zaid, M.H.M., et al.: Influence of heavy metal oxides to the mechanical and radiation shielding properties of borate and silica glass system. J. Mater. Res. Technol. 11, 1322–1330 (2021)
Gerward, L., et al.: WinXCom–a program for calculating X-ray attenuation coefficients. Radiat. phys. chem. 71(3–4), 653–654 (2004)
Murty, R.: Effective atomic numbers of heterogeneous materials. Nature 207(4995), 398–399 (1965)
Mayneord, W.: The significance of the roentgen. Acta Int Union Against Cancer 2, 271 (1937)
Hine, G.J.: Secondary electron emission and effective atomic numbers. Nucleonics (US) Ceased publication, 10 (1952)
Tsai, C.; Cho, Z.: Physics of contrast mechanism and averaging effect of linear attenuation coefficients in a computerized transverse axial tomography (CTAT) transmission scanner. Phys. Med. Biol. 21(4), 544 (1976)
Sellakumar, P.; Samuel, E.J.; Supe, S.S.: Water equivalence of polymer gel dosimeters. Radiat. Phys. Chem. 76, 1108–1115 (2007)
Puumalainen, P.; Olkkonen, H.; Sikanen, P.: Assessment of fat content of liver by a photon scattering technique. Int. J. Appl. Radiat. Isotope. 28(9), 785–787 (1977)
Manninen, S.; Koikkalainen, S.: Determination of the effective atomic number using elastic and inelastic scattering of γ-rays. Int. J. Appl. Radiat. Isotope. 35(10), 965–968 (1984)
El-Khayatt, A.M.: Calculation of photon shielding properties for some neutron shielding materials. Nucl. Sci. Tech. 28(5), 69 (2017)
Manohara, S.R.; Hanagodimath, S.M.; Gerward, L.: Photon interaction and energy absorption in glass: a transparent gamma ray shield. J. Nucl. Mater. 393(3), 465–472 (2009)
Sikora, P., et al.: Evaluation of the effects of bismuth oxide (Bi2O3) micro and nanoparticles on the mechanical, microstructural and γ-ray/neutron shielding properties of Portland cement pastes. Constr. Build. Mater. 284, 122758 (2021)
El-Khayatt, A.M.; Al-Rajhi, M.A.; Hassan, A.T.; Idriss, H.: Improvement of shielding properties of cement paste by slag addition from local steel industry. J. Test. Eval. 49(5), 3444–3459 (2021)
Gökçe, H.S.; Güngör, O.; Yılmaz, H.: An online software to simulate the shielding properties of materials for neutrons and photons: NGCal. Radiat. Phys. Chem. 185, 109519 (2021)
El-Khayatt, A.M.: NXcom—A program for calculating attenuation coefficients of fast neutrons and gamma-rays. Annal. Nucl. Energy 38(1), 128–132 (2011)
Kaplan, M.F.: Concrete radiation shielding, p. 476. Longman Scientific and Technical, London (1989)
Libeesh, N.K., et al.: Applicability of the multispectral remote sensing on determining the natural rock complexes distribution and their evaluability on the radiation protection applications. Radiat. Phys. Chem. 193, 110004 (2022)
Bashter, I.I.; El-Sayed Abdo, A.; Makarious, A.S.: A comparative study of the attenuation of reactor thermal neutrons in different types of concrete. Annal. Nucl. Energy 23, 1189–1195 (1996)
Mahmoud, K.A.; Sayyed, M.I.; Tashlykov, O.L.: Comparative studies between the shielding parameters of concretes with different additive aggregates using MCNP-5 simulation code. Radiat. Phys. Chem. 165, 108426 (2019)
Ozyurt, O., et al.: Calculation of gamma ray attenuation coefficients of some granite samples using a Monte Carlo simulation code. Radiat. Phys. Chem. 144, 271–275 (2018)
Mahmoud, K.A., et al.: Investigation of the gamma ray shielding properties for polyvinyl chloride reinforced with chalcocite and hematite minerals. Heliyon 6(3), e03560 (2020)
Mahmoud, K.A., et al.: A new heavy-mineral doped clay brick for gamma-ray protection purposes. Appl. Radiat. Isotope. 173, 109720 (2021)
Sallam, O.I.; Madbouly, A.M.; Ezz-Eldin, F.M.: Impact of Nd3+ additive on the radiation shielding competence of borosilicate glasses fabricated from agro-waste materials. J. Non-Cryst. Sol. 590, 121691 (2022)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.A.M. and A.M.E.-K: Conceptualization. A.M.E.-K. and K.A.M: Software. M.A.M., A.M.Z. and A.M.E.-K: Validation. A.M.E.-K. and K.A.M: Formal analysis. M.A.M., A.M.E.-K, and K.A.M: Data curation. M.A.M., K.A.M., and A.M.E.-K: Writing—original draft. M.A.M, A.M.Z., P.P., K.A.M., and A.M.E.-K: Writing—review and editing. P.P., M.A.M., and A.M.E.-K: Visualization. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zayed, A.M., El-Khayatt, A.M., Mahmoud, K.A. et al. Evaluation of Some Heavyweight Minerals as Sustainable Neutron and Gamma-Ray Attenuating Materials: Comprehensive Theoretical and Simulation Investigations. Arab J Sci Eng (2024). https://doi.org/10.1007/s13369-024-09300-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13369-024-09300-2