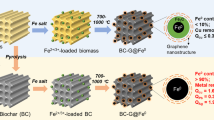
Abstract
The treatment of boron in fracturing backflow fluid is a widely studied issue in oil and gas resource extraction. In this work, the material of canola straw-derived biochar composite graphene oxide (BC@GO) was produced and used for boron removal. Then, a series of characterization and batch adsorption experiments were conducted on the material. The results show that the composite material has good adsorption capacity, and the maximum adsorption capacity is 168mg/g at the initial concentration of 300 mg/L at pH = 7. The results of kinetic and thermodynamic fitting showed that the boron adsorption by BC@GO conforms to the pseudo-second-order kinetics model and Freundlich model. In addition, it was found that the material still has a good adsorption capacity in batch adsorption experiments of hydraulic fracturing simulated water. Therefore, BC@GO is a new boron removal material with good application prospects.












Similar content being viewed by others
References
Thomas, L.; Tang, H.; Kalyon, D.M.; Aktas, S.; Arthur, J.D.; Blotevogel, J.; Carey, J.W.; Filshill, A.; Fu, P.; Hsuan, G.; Hu, T.; Soeder, D.; Shah, S.; Vidic, R.D.; Young, M.H.: Toward better hydraulic fracturing fluids and their application in energy production: a review of sustainable technologies and reduction of potential environmental impacts. J. Petrol. Sci. Eng. 173, 793–803 (2019). https://doi.org/10.1016/j.petrol.2018.09.056
You, Q.; Wang, H.; Zhang, Y.; Liu, Y.; Fang, J.; Dai, C.: Experimental study on spontaneous imbibition of recycled fracturing flow-back fluid to enhance oil recovery in low permeability sandstone reservoirs. J. Petrol. Sci. Eng. 166, 375–380 (2018). https://doi.org/10.1016/j.petrol.2018.03.058
Yilmaz, A.E.; Boncukcuoğlu, R.; Bayar, S.; Fil, B.A.; Kocakerim, M.M.: Boron removal by means of chemical precipitation with calcium hydroxide and calcium borate formation. Korean J. Chem. Eng. 29, 1382–1387 (2012). https://doi.org/10.1007/s11814-012-0040-1
Zhang, Z.; Mao, J.; Zhang, Y.; Han, T.; Yang, B.; Xiao, W.: Improved fracturing fluid using: organic amino boron composite crosslinker with BN covalent bond. J of Applied Polymer Sci 136, 47675 (2019). https://doi.org/10.1002/app.47675
Peng, X.; Shi, D.; Zhang, Y.; Zhang, L.; Ji, L.; Li, L.: Recovery of boron from unacidified salt lake brine by solvent extraction with 2,2,4-trimethyl-1,3-pentanediol. J. Mol. Liq. 326, 115301 (2021). https://doi.org/10.1016/j.molliq.2021.115301
The 5th annual international conference on material engineering and application. In: IOP Conference Series: Materials and Science and Engineering, vol. 484. pp. 011001 (2019). https://doi.org/10.1088/1757-899X/484/1/011001.
Kluczka, J.; Trojanowska, J.; Zolotajkin, M.; Ciba, J.; Turek, M.; Dydo, P.: Boron removal from wastewater using adsorbents. Environ. Technol. 28, 105–113 (2007). https://doi.org/10.1080/09593332808618769
Yandri, Y.; Ropingi, H.; Suhartati, T.; Irawan, B.; Hadi, S.: Immobilization of Aspergillus fumigatus α-amylase via adsorption onto bentonite/chitosan for stability enhancement. Emerg Sci J 7, 811–1826 (2023). https://doi.org/10.28991/ESJ-2023-07-05-023
Yandri, Y.; Tiarsa, E.R.; Suhartati, T.; Irawan, B.; Hadi, S.: Immobilization and stabilization of Aspergillus Fumigatus α-amylase by adsorption on a chitin. Emerg Sci J 7, 77–89 (2022). https://doi.org/10.28991/ESJ-2023-07-01-06
Aneke, F.; Adu, J.: Adsorption of heavy metals from contaminated water using Leachate modular tower. Civ Eng J 9, 1522–1541 (2023). https://doi.org/10.28991/CEJ-2023-09-06-017
Lin, J.-Y.; Mahasti, N.N.N.; Huang, Y.-H.: Recent advances in adsorption and coagulation for boron removal from wastewater: a comprehensive review. J. Hazard. Mater. 407, 124401 (2021). https://doi.org/10.1016/j.jhazmat.2020.124401
Zelmanov, G.; Semiat, R.: Boron removal from water and its recovery using iron (Fe+3) oxide/hydroxide-based nanoparticles (NanoFe) and NanoFe-impregnated granular activated carbon as adsorbent. Desalination 333, 107–117 (2014). https://doi.org/10.1016/j.desal.2013.11.027
Kluczka, J.; Korolewicz, T.; Zołotajkin, M.; Simka, W.; Raczek, M.: A new adsorbent for boron removal from aqueous solutions. Environ. Technol. 34, 1369–1376 (2013). https://doi.org/10.1080/09593330.2012.750380
Myachina, M.A.; Polyakova, Yu.A.; Gavrilova, N.N.; Nazarov, V.V.; Kolesnikov, V.A.: ZrO2–Carbon nanotubes composite sorbent for treatment of aqueous solutions to remove boron. Russ. J. Appl. Chem. 90, 895–900 (2017). https://doi.org/10.1134/S107042721706009X
Le, Y.; Guan, Y.; Ma, X.; Zhang, W.: Preparation and boron removal performance of glycidol modified PANI nanorods: an optimization study based on response surface methodology. Polymers 15, 459 (2023). https://doi.org/10.3390/polym15020459
Dong, H.; Wang, S.; Niu, S.; Sha, X.; Ji, Z.; Wang, X.; Zhang, X.: Preparation and application of porous functional polymers for boron removal in seawater desalination by a mild and free-organic solvents process. Desalination 560, 116658 (2023). https://doi.org/10.1016/j.desal.2023.116658
Sun, L.; Huang, J.; Liu, H.; Zhang, Y.; Ye, X.; Zhang, H.; Wu, A.; Wu, Z.: Adsorption of boron by CA@KH-550@EPH@NMDG (CKEN) with biomass carbonaceous aerogels as substrate. J. Hazard. Mater. 358, 10–19 (2018). https://doi.org/10.1016/j.jhazmat.2018.06.040
Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rosso, C.; Rovere, M.; Tagliaferro, A.: Unraveling the biochar potential as filler for composites preparation. Macromol. Symp. 408, 2200085 (2023). https://doi.org/10.1002/masy.202200085
Sui, L.; Tang, C.; Du, Q.; Zhao, Y.; Cheng, K.; Yang, F.: Preparation and characterization of boron-doped corn straw biochar: Fe(II) removal equilibrium and kinetics. J. Environ. Sci. 106, 116–123 (2021). https://doi.org/10.1016/j.jes.2021.01.001
Jiang, C.; Bo, J.; Xiao, X.; Zhang, S.; Wang, Z.; Yan, G.; Wu, Y.; Wong, C.; He, H.: Converting waste lignin into nano-biochar as a renewable substitute of carbon black for reinforcing styrene-butadiene rubber. Waste Manage. 102, 732–742 (2020). https://doi.org/10.1016/j.wasman.2019.11.019
Xue, B.; Wang, X.; Sui, J.; Xu, D.; Zhu, Y.; Liu, X.: A facile ball milling method to produce sustainable pyrolytic rice husk bio-filler for reinforcement of rubber mechanical property. Ind. Crops Prod. 141, 111791 (2019). https://doi.org/10.1016/j.indcrop.2019.111791
Lin, M.; Li, F.; Li, X.; Rong, X.; Oh, K.: Biochar-clay, biochar-microorganism and biochar-enzyme composites for environmental remediation: a review. Environ. Chem. Lett. 21, 1837–1862 (2023). https://doi.org/10.1007/s10311-023-01582-6
Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V.: Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: a meta-analysis. Biochar 4, 8 (2022). https://doi.org/10.1007/s42773-022-00138-1
Rodríguez-Narciso, S.; Lozano-Álvarez, J.A.; Salinas-Gutiérrez, R.; Castañeda-Leyva, N.: A Stochastic model for adsorption kinetics. Adsorpt. Sci. Technol. 2021, 5522581 (2021). https://doi.org/10.1155/2021/5522581
Xu, C.; Yuan, R.; Wang, X.: Selective reduction of graphene oxide. New Carbon Mater. 29, 61–66 (2014). https://doi.org/10.1016/S1872-5805(14)60126-8
Chen, F.; Guo, L.; Zhang, X.; Yi Leong, Z.; Yang, S.; Ying Yang, H.: Nitrogen-doped graphene oxide for effectively removing boron ions from seawater. Nanoscale 9, 326–333 (2017). https://doi.org/10.1039/C6NR07448K
Al-Afy, N.; Sereshti, H.: Rapid removal of boron from environmental water samples using magnetic graphene oxide: optimized by central composite design. DWT 153, 65–75 (2019). https://doi.org/10.5004/dwt.2019.23948
Amrutha, G.; Jeppu, C.R.; Girish, B.; Prabhu, K.: Mayer, multi-component adsorption isotherms: review and modeling studies. Environ. Process. 10, 38 (2023). https://doi.org/10.1007/s40710-023-00631-0
Yuan, R.; Si, T.; Lu, Q.; Bian, R.; Wang, Y.; Liu, X.; Zhang, X.; Zheng, J.; Cheng, K.; Joseph, S.; Li, L.; Pan, G.: Rape straw biochar enhanced Cd immobilization in flooded paddy soil by promoting Fe and sulfur transformation. Chemosphere 339, 139652 (2023). https://doi.org/10.1016/j.chemosphere.2023.139652
Zhu, X.; Shen, J.; Kang, J.; Yan, P.; Yuan, L.; Cheng, Y.; Wang, B.; Zhao, S.; Chen, Z.: Surface atomic oxygen species mediated the in-situ formation of hydroxyl radicals on Fe3C decorated biochar for enhancing catalytic ozonation. Chem. Eng. J. 473, 145380 (2023). https://doi.org/10.1016/j.cej.2023.145380
Palansooriya, K.N.; Yoon, I.-H.; Kim, S.-M.; Wang, C.-H.; Kwon, H.; Lee, S.-H.; Igalavithana, A.D.; Mukhopadhyay, R.; Sarkar, B.; Ok, Y.S.: Designer biochar with enhanced functionality for efficient removal of radioactive cesium and strontium from water. Environ. Res. 214, 114072 (2022). https://doi.org/10.1016/j.envres.2022.114072
Li, B.; Yang, L.; Wang, C.; Zhang, Q.; Liu, Q.; Li, Y.; Xiao, R.: Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 175, 332–340 (2017). https://doi.org/10.1016/j.chemosphere.2017.02.061
Bicak, N.; Gazi, M.; Senkal, B.F.: Polymer supported amino bis-(cis-propan 2,3 diol) functions for removal of trace boron from water. React. Funct. Polym. 65, 143–148 (2005). https://doi.org/10.1016/j.reactfunctpolym.2005.01.010
Wu, Q.; Liu, M.; Wang, X.: A novel chitosan based adsorbent for boron separation. Sep. Purif. Technol. 211, 162–169 (2019). https://doi.org/10.1016/j.seppur.2018.09.070
Xu, L.; Liu, Y.; Hu, H.; Wu, Z.; Chen, Q.: Synthesis, characterization and application of a novel silica based adsorbent for boron removal. Desalination 294, 1–7 (2012). https://doi.org/10.1016/j.desal.2012.02.030
Harada, A.; Takagi, T.; Kataoka, S.; Yamamoto, T.; Endo, A.: Boron adsorption mechanism on polyvinyl alcohol. Adsorption 17, 171–178 (2011). https://doi.org/10.1007/s10450-010-9300-8
Gazi, M.; Galli, G.; Bicak, N.: The rapid boron uptake by multi-hydroxyl functional hairy polymers. Sep. Purif. Technol. 62, 484–488 (2008). https://doi.org/10.1016/j.seppur.2008.02.004
Morisada, S.; Rin, T.; Ogata, T.; Kim, Y.-H.; Nakano, Y.: Adsorption removal of boron in aqueous solutions by amine-modified tannin gel. Water Res. 45, 4028–4034 (2011). https://doi.org/10.1016/j.watres.2011.05.010
Gao, Z.; Xie, S.; Zhang, B.; Qiu, X.; Chen, F.: Ultrathin Mg–Al layered double hydroxide prepared by ionothermal synthesis in a deep eutectic solvent for highly effective boron removal. Chem. Eng. J. 319, 108–118 (2017). https://doi.org/10.1016/j.cej.2017.03.002
Demirçivi, P.; Saygılı, G.N.: Comparative study of modified expanded perlite with hexadecyltrimethylammonium-bromide and gallic acid for boron adsorption. J. Mol. Liq. 254, 383–390 (2018). https://doi.org/10.1016/j.molliq.2018.01.116
Liao, X.; Wang, B.; Zhang, Q.: Synthesis of glycopolymer nanosponges with enhanced adsorption performances for boron removal and water treatment. J. Mater. Chem. A 6, 21193–21206 (2018). https://doi.org/10.1039/C8TA06802J
Acknowledgements
This research was supported by the Opening Project of Oil and Gas Field Applied Chemistry Key Laboratory of Sichuan Province (YQKF202108) awarded to ML, and the University Students Innovation and Entrepreneurship Training Program (202210615040) awarded to XW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, X., Luo, M., Wang, T. et al. Preparation of Biochar Composite Graphene Oxide for the Removal of Boron in Simulated Fracturing Flowback Fluid. Arab J Sci Eng (2024). https://doi.org/10.1007/s13369-024-09126-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13369-024-09126-y