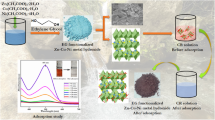
Abstract
Clean water is the basic need of every living organism. The field of nanotechnology is one of the utmost wide spread areas for current research and development for the management of discarded water. Zinc oxide, iron oxide and copper oxide nanoparticles were synthesized using Punica granatum leave, and pulp extract. These prepared nanoparticles were applied for the removal of anionic toxic dyes from wastewater using batch experiment. Different parameters like pH, dose, initial dye concentration, contact time and temperature were optimized to check the highest removal of selected dye. The effect of presence of electrolytes was also studied. Kinetic models like pseudo-1st-order, pseudo-2nd-order and intraparticle diffusion model were applied to check the rate and order of reaction. Equilibrium models like Freundlich, Langmuir, Temkin and Harkins–Jura were applied to check the nature of adsorption of dye on prepared nanoparticles. Thermodynamics models were also applied to check the enthalpy, entropy and Gibbs free energy of the reaction. Desorption study was conducted to check the reusability of the nanoparticles.











Similar content being viewed by others
Availability of Data and Materials
Not applicable.
References
Naiya, T.K.; Bhattacharya, A.K.; Das, S.K.: Adsorption of Cd (II) and Pb (II) from aqueous solutions on activated alumina. J. Colloid Interface Sci. 333(1), 14–26 (2009)
Rasheed, T.: Covalent organic frameworks as promising adsorbent paradigm for environmental pollutants from aqueous matrices: perspective and challenges. Sci. Total Environ. 833, 155279 (2022)
Munjur, H.M., et al.: Biodegradable natural carbohydrate polymeric sustainable adsorbents for efficient toxic dye removal from wastewater. J. Mol. Liq. 319, 114356 (2020)
Hasan, M.M., et al.: Sustainable ligand-modified based composite material for the selective and effective cadmium (II) capturing from wastewater. J. Mol. Liq. 371, 121125 (2023)
Zhao, F., et al.: New insights into eutrophication management: importance of temperature and water residence time. J. Environ. Sci. 111, 229–239 (2022)
Mazrouaa, A.M., et al.: Nano-composite multi-wall carbon nanotubes using poly (p-phenylene terephthalamide) for enhanced electric conductivity. J. Environ. Chem. Eng. 7(2), 103002 (2019)
Islam, A., et al.: Assessment of clean H2 energy production from water using novel silicon photocatalyst. J. Clean. Prod. 244, 118805 (2020)
Ihsanullah, I.; Bilal, M.; Jamal, A.: Recent developments in the removal of dyes from water by starch-based adsorbents. Chem. Rec. 22, e202100312 (2022)
Rasheed, T., et al.: Nano and micro architectured cues as smart materials to mitigate recalcitrant pharmaceutical pollutants from wastewater. Chemosphere 274, 129785 (2021)
Zubair, M., et al.: Adsorption and reusability performance of M-Fe (M= Co, Cu, Zn and Ni) layered double hydroxides for the removal of hazardous Eriochrome Black T dye from different water streams. J. Water Process Eng. 42, 102060 (2021)
Ali, I.; Gupta, V.: Advances in water treatment by adsorption technology. Nat. Protoc. 1(6), 2661 (2006)
Bakather, O.Y., et al.: Enhanced adsorption of selenium ions from aqueous solution using iron oxide impregnated carbon nanotubes. Bioinorg. Chem. Appl. 2017, 1–12 (2017)
Ihsanullah, C.: nanotube membranes for water purification: developments, challenges, and prospects for the future. Sep. Purif. Technol. 209, 307–337 (2019)
Naqvi, S.T.R., et al.: Modification strategies for improving the solubility/dispersion of carbon nanotubes. J. Mol. Liq. 297, 111919 (2020)
Rasheed, T., et al.: Carbon nanotubes assisted analytical detection—sensing/delivery cues for environmental and biomedical monitoring. TrAC Trends Anal. Chem. 132, 116066 (2020)
Rasheed, T., et al.: Carbon nanotubes-based cues: a pathway to future sensing and detection of hazardous pollutants. J. Mol. Liq. 292, 111425 (2019)
Hasan, M.M., et al.: Natural biodegradable polymeric bioadsorbents for efficient cationic dye encapsulation from wastewater. J. Mol. Liq. 323, 114587 (2021)
Teo, S.H., et al.: Sustainable toxic dyes removal with advanced materials for clean water production: a comprehensive review. J. Clean. Prod. 332, 130039 (2022)
Kubra, K.T., et al.: Efficient encapsulation of toxic dye from wastewater using biodegradable polymeric adsorbent. J. Mol. Liq. 329, 115541 (2021)
Kubra, K.T.; Salman, M.S.; Hasan, M.N.: Enhanced toxic dye removal from wastewater using biodegradable polymeric natural adsorbent. J. Mol. Liq. 328, 115468 (2021)
Bilal, M., et al.: Agarose-chitosan hydrogel-immobilized horseradish peroxidase with sustainable bio-catalytic and dye degradation properties. Int. J. Biol. Macromol. 124, 742–749 (2019)
Elkhider, K.H., et al.: Synthesis, characterization and dye adsorption performance of strontium ferrite decorated bentonite-CoNiAl magnetic composite. Arab. J. Sci. Eng. 45(9), 7397–7408 (2020)
Rasheed, T., et al.: Reaction mechanism and degradation pathway of rhodamine 6G by photocatalytic treatment. Water Air Soil Pollut. 228(8), 1–10 (2017)
Rasheed, T., et al.: TiO2/UV-assisted rhodamine B degradation: putative pathway and identification of intermediates by UPLC/MS. Environ. Technol. 39(12), 1533–1543 (2018)
Rasheed, T., et al.: Biogenic synthesis and characterization of cobalt oxide nanoparticles for catalytic reduction of direct yellow-142 and methyl orange dyes. Biocatal. Agric. Biotechnol. 19, 101154 (2019)
Brookstein, D.S.: Factors associated with textile pattern dermatitis caused by contact allergy to dyes, finishes, foams, and preservatives. Dermatol. Clin. 27(3), 309–322 (2009)
Naushad, M., et al.: Adsorption of textile dye using para-aminobenzoic acid modified activated carbon: kinetic and equilibrium studies. J. Mol. Liq. 296, 112075 (2019)
Znad, H., et al.: Synthesis a novel multilamellar mesoporous TiO2/ZSM-5 for photo-catalytic degradation of methyl orange dye in aqueous media. J. Environ. Chem. Eng. 6(1), 218–227 (2018)
Özkan, K.; Yıldız, K.; Yağli, H.: Is Kalina cycle or organic Rankine cycle for industrial waste heat recovery applications? A detailed performance, economic and environment based comprehensive analysis. Process Saf. Environ. Prot. 163, 421–437 (2022)
Royer, B., et al.: Applications of Brazilian pine-fruit shell in natural and carbonized forms as adsorbents to removal of methylene blue from aqueous solutions—kinetic and equilibrium study. J. Hazard. Mater. 164(2–3), 1213–1222 (2009)
Awual, M.R.: Novel nanocomposite materials for efficient and selective mercury ions capturing from wastewater. Chem. Eng. J. 307, 456–465 (2017)
Bora, A., et al.: Microbes incorporated nanomaterials for water purification. In: Handbook of Microbial Nanotechnology, pp. 439–459. Elsevier (2022)
Hofman-Caris, R.C., et al.: Removal of nanoparticles (both inorganic nanoparticles and nanoplastics) in drinking water treatment–coagulation/flocculation/sedimentation, and sand/granular activated carbon filtration. Environ. Sci. Water Res. Technol. 8, 1675–1686 (2022)
Wei, K.-H., et al.: Recent progress on in-situ chemical oxidation for the remediation of petroleum contaminated soil and groundwater. J. Hazard. Mater. 432, 128738 (2022)
Sathish, T.; Saravanan, R.; Vijayan, V.: Investigations on influences of MWCNT composite membranes in oil refineries waste water treatment with Taguchi route. Chemosphere 298, 134265 (2022)
Rajendran, S., et al.: Generation of novel npn (CeO2-PPy-ZnO) heterojunction for photocatalytic degradation of micro-organic pollutants. Environ. Pollut. 292, 118375 (2022)
Yeamin, M.B., et al.: Efficient encapsulation of toxic dyes from wastewater using several biodegradable natural polymers and their composites. J. Clean. Prod. 291, 125920 (2021)
Gao, H., et al.: Removal of anionic azo dyes from aqueous solution using magnetic polymer multi-wall carbon nanotube nanocomposite as adsorbent. Chem. Eng. J. 223, 84–90 (2013)
Rasheed, T., et al.: Organic covalent interaction-based frameworks as emerging catalysts for environment and energy applications: current scenario and opportunities. Chem. Asian J. 18, e202300196 (2023)
Rasheed, T.; Anwar, M.T.: Metal organic frameworks as self-sacrificing modalities for potential environmental catalysis and energy applications: challenges and perspectives. Coord. Chem. Rev. 480, 215011 (2023)
Rasheed, T.: Carbon dots as robust class of sustainable and environment friendlier nano/optical sensors for pesticide recognition from wastewater. TrAC Trends Anal. Chem. 160, 116957 (2023)
Rasheed, T.: Carbon dots as potential greener and sustainable fluorescent nanomaterials in service of pollutants sensing. TrAC Trends Anal. Chem. 158, 116841 (2022)
Bhaumik, M., et al.: Removal of fluoride from aqueous solution by polypyrrole/Fe3O4 magnetic nanocomposite. J. Hazard. Mater. 186(1), 150–159 (2011)
Aksakal, O.; Ucun, H.: Equilibrium, kinetic and thermodynamic studies of the biosorption of textile dye (Reactive Red 195) onto Pinus sylvestris L. J. Hazard. Mater. 181(1–3), 666–672 (2010)
Das, M.C., et al.: A Zn4O-containing doubly interpenetrated porous metal–organic framework for photocatalytic decomposition of methyl orange. Chem. Commun. 47(42), 11715–11717 (2011)
Basheer, S., et al.: Oil characterization and elemental analysis of the seeds of Punica granatum L. J. Fac. Sci. Technol. 9(1), 64–72 (2022)
Bala, N., et al.: Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 5(7), 4993–5003 (2015)
Naseer, M., et al.: Green route to synthesize zinc oxide nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 10(1), 9055 (2020)
Devatha, C.; Thalla, A.K.; Katte, S.Y.: Green synthesis of iron nanoparticles using different leaf extracts for treatment of domestic waste water. J. Clean. Prod. 139, 1425–1435 (2016)
Mahmoud, A.E.D., et al.: Green copper oxide nanoparticles for lead, nickel, and cadmium removal from contaminated water. Sci. Rep. 11(1), 12547 (2021)
Charumathi, D.; Das, N.: Packed bed column studies for the removal of synthetic dyes from textile wastewater using immobilised dead C. tropicalis. Desalination 285, 22–30 (2012)
Low, K.; Lee, C.: The removal of cationic dyes using coconut husk as an adsorbent. Pertanika 13(2), 221–228 (1990)
Essandoh, M.; Garcia, R.A.: Efficient removal of dyes from aqueous solutions using a novel hemoglobin/iron oxide composite. Chemosphere 206, 502–512 (2018)
Al-Degs, Y.S., et al.: Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigm. 77(1), 16–23 (2008)
Islam, A., et al.: Step towards the sustainable toxic dyes removal and recycling from aqueous solution-a comprehensive review. Resour. Conserv. Recycl. 175, 105849 (2021)
Zafar, M.N., et al.: Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol 8(1), 713–725 (2019)
Yavari, S., et al.: Cobalt ferrite nanoparticles: preparation, characterization and anionic dye removal capability. J. Taiwan Inst. Chem. Eng. 59, 320–329 (2016)
Siddique, R., et al.: Properties of bacterial rice husk ash concrete. Constr. Build. Mater. 121, 112–119 (2016)
Nairat, M.; Shahwan, T.; Eroğlu, A.E.; Fuchs, H.: Incorporation of iron nanoparticles into clinoptilolite and its application for the removal of cationic and anionic dyes. J. Ind. Eng. Chem. 21, 1143–1151 (2015)
Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.; Hook, J.M.; Antill, S.J.; Kepert, C.J.: Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane. J. Phys. Chem. C, 112(40), 15742–15751 (2008)
Kale, R.D.; Kane, P.B.: Colour removal of phthalocyanine based reactive dye by nanoparticles. Groundwater Sustain. Dev. 8, 309–318 (2019)
Abagero, W.B.: Exploring the potentialities of waste plant materials for the production of gold nanoparticles and multi-metallic composite particles and its application in wastewater treatment (Doctoral dissertation, Universidade do Algarve (Portugal)) (2017)
Hsu, A.; Zomer, A.: Environmental performance index. Wiley StatsRef: Statistics Reference Online, 1-5 (2014)
Foroutan-Nejad, C.; Novák, M.; Marek, R.: Comment on some unexpected behavior of the adsorption of alkali metal ions onto the graphene surface under the effect of external electric field. J. Phys. Chem. C, 119(10), 5752–5754 (2015)
Jayanthi, K.; Rao, N.B.; Murali, P.V.: Physico-chemical status of water samples collected from rampally lake near Ecil Hyderabad city. Ann. Rom. Soc. Cell Biol. 5896–5906 (2021)
Kumari, P.; Parashara, H.: Β-cyclodextrin modified magnetite nanoparticles for efficient removal of eosin and phloxine dyes from aqueous solution. Mater. Today: Proceedings 5(7), 15473–15480 (2018)
Tambat, S.N.; Sane, P.K.; Suresh, S.; Varadan, N.; Pandit, A.B.; Sontakke, S.M.: Hydrothermal synthesis of NH2-UiO-66 and its application for adsorptive removal of dye. Adv. Powder Technol. 29(11), 2626–2632 (2018)
Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V.: Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 7, 1831 (2016)
Venkatesh, M.; Gouthaman, S.; Kanemoto, S.O.; Lakshmi, M.S.; Hamerton, I.: Development of epoxy-cyanate ester-clay nanocomposites offering enhanced thermally stability. J. Appl. Polym. Sci. 136(28), 47754 (2019). https://doi.org/10.1002/app.47754
Sang, Y.; Zhao, Z.; Zhao, M.; Hao, P.; Leng, Y.; Liu, H.: From UV to near-infrared, WS2 nanosheet: a novel photocatalyst for full solar light spectrum photodegradation. Adv. Mater. 27(2), 363–369 (2015)
Zafar, M.N.; Dar, Q.; Nawaz, F.; Zafar, M.N.; Iqbal, M.; Nazar, M.F.: Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 8(1), 713–725 (2019)
Aksu, Z.; Balibek, E.: Effect of salinity on metal-complex dye biosorption by Rhizopus arrhizus. J. Environ. Manag. 91(7), 1546–1555 (2010)
Banisheykholeslami, F.; et al.: Design of novel hyper-branched dendritic boehmite/gallic acid alumoxane for methylene blue removal: adsorption mechanism and reusability. Korean J. Chem. Eng. 40(4), 841–853 (2023)
Soliman, A.I.; Abdel-Wahab, A.-M.A.; Abdelhamid, H.N.: Hierarchical porous zeolitic imidazolate frameworks (ZIF-8) and ZnO@ N-doped carbon for selective adsorption and photocatalytic degradation of organic pollutants. RSC Adv. 12(12), 7075–7084 (2022)
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40(9), 1361–1403 (1918)
Funding
Authors did not receive any funding for this research.
Author information
Authors and Affiliations
Contributions
Conceptualization, SA, GM, TR; writing—original draft preparation, SA, MGM; writing—review and editing, MR, DNA, AA, TR, revisions and final editing, SK, TR. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abid, S., Mustafa, G., Rizwan, M. et al. Synthesis of Metal Oxide Nanoparticles Using Punica granatum Extract for the Removal of Cationic and Anionic Dyes from Wastewater. Arab J Sci Eng 49, 515–530 (2024). https://doi.org/10.1007/s13369-023-08166-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-08166-0