Abstract
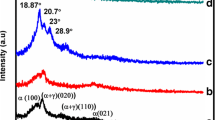
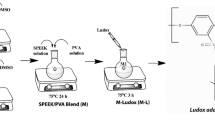
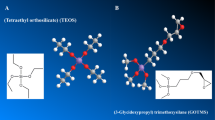
Present work demonstrates development and characterization of Nexar-based nanocomposite membranes to evaluate their potential as an alternative to Nafion. Nanocomposite membranes are characterized through XRD, FTIR, SEM, and TGA for crystallinity, surface morphology, functionalities, and thermal stability. Membrane thickness, and protonic resistivity are determined. Modification of Nexar with doped silica and sulfonated silica nanoparticles is checked for water uptake (UR), swelling ratio (SR), ion exchange capacity, proton conductivity at different temperatures, humidity, and activation energies. Incorporating silica and functionalized silica into Nexar drastically changed WU and SR. Proton conductivity improved by 58.8% for sulfonated silica membrane, comparing pristine Nexar. At low humidity, composite membranes showed better proton conductivity than pristine Nexar. Low activation energies supported mainly Grotthus mechanism for proton transport. Thus, present work suggests Nexar-based functionalized nanocomposite membranes owing to higher IEC and less swelling ratio may help higher proton conductivity necessary for PEM fuel cell operation.











Similar content being viewed by others
Abbreviations
- PEM:
-
Proton exchange membrane
- IEC:
-
Ion exchange capacity
- PBC:
-
Pentablock copolymer
- SiO2 :
-
Silicon dioxide (Silica)
- SiO2–SO3H:
-
Sulfonated silicon dioxide (Silica)
- SPAES:
-
Sulfonated polyarylene ether ketones
- SSEBS:
-
Sulfonated polystyrene ethylene butylene polystyrene
- PBI:
-
Polybenzimidazole
- PVDF:
-
Polyvinylidiene fluoride
- WU:
-
Water uptake
- SR:
-
Swelling ratio
- GO:
-
Graphene oxide
- XRD:
-
X-ray powder diffraction
- FTIR:
-
Fourier transform infrared spectroscopy
- SEM:
-
Scanning electron microscopy
- TGA:
-
Thermogravimetric analysis
- NPs:
-
Nanoparticles
References
Xu, G.; Wu, Z.; Wei, Z.; Zhang, W.; Wu, J.; Li, Y.; Li, J.; Qu, K.; Cai, W.: Non-destructive fabrication of Nafion/silica composite membrane via swelling-filling modification strategy for high temperature and low humidity PEM fuel cell. Renew. Energy. 153, 935–939 (2020). https://doi.org/10.1016/j.renene.2020.02.056
Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R.: The role of renewable energy in the global energy transformation. Energy Strateg. Rev. 24, 38–50 (2019). https://doi.org/10.1016/j.esr.2019.01.006
I.E.A.: The impacts of the Covid 19 crisis on global energy demand and CO2 emissions, Global Energy Review. (2020)
Shaari, N.; Kamarudin, S.K.: Recent advances in additive-enhanced polymer electrolyte membrane properties in fuel cell applications: an overview. Int. J. Energy Res. 43, 2756–2794 (2019). https://doi.org/10.1002/er.4348
Raduwan, N.F.; Shaari, N.; Kamarudin, S.K.; Masdar, M.S.; Yunus, R.M.: An overview of nanomaterials in fuel cells: synthesis method and application. Int. J. Hydrog. Energy. 47, 18468–18495 (2022). https://doi.org/10.1016/j.ijhydene.2022.03.035
Hussain, M.M.; Dincer, I.; Li, X.: A preliminary life cycle assessment of PEM fuel cell powered automobiles. Appl. Therm. Eng. 27, 2294–2299 (2007). https://doi.org/10.1016/j.applthermaleng.2007.01.015
Luo, Y.; Shi, Y.; Cai, N.: Distributed hybrid system and prospect of the future Energy Internet. In: Reading, L. (Ed.) Hybrid systems and multi-energy networks for the future energy internet, pp. 9–39. Academic Press, Cambridge (2021). https://doi.org/10.1016/B978-0-12-819184-2.00002-X
Wang, Y.; Chen, K.S.; Mishler, J.; Chan, S.C.; Cordobes, X.C.: A review of polymer electrolyte membrane fuel cells : technology, applications, and needs on fundamental research. Appl. Energy. 88, 981–1007 (2011). https://doi.org/10.1016/j.apenergy.2010.09.030
Veziroğlu, T.N.: Foreword. In: Veziroğlu, T.N. (Ed.) PEM fuel cells, pp. 9–10. Elsevier, Hoboken (2013)
Feroldi, D.; Basualdo, M.: Description of PEM fuel cells system. In: Basualdo, M.S.; Feroldi, D.; Outbib, R. (Eds.) PEM fuel cells with bio-ethanol processor systems. Springer, London (2012)
Behling, N.H.: Strengths and weaknesses of major government fuel cell R&D programs. In: Behling, N.H. (Ed.) Fuel Cells, pp. 601–619. Elsevier, Hoboken (2013)
Zhang, J.; Zhang, H.; Wu, J.; Zhang, J.: PEM fuel cell fundamentals. In: Zhang, J. (Ed.) PEM fuel cell testing and diagnosis, pp. 1–42. Elsevier, Hoboken (2013)
Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C.: Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today. 32, 178–203 (2020). https://doi.org/10.1016/j.mattod.2019.06.005
Wu, W.; Zhai, C.; Sui, Z.; Sui, Y.; Luo, X.: Proton exchange membrane fuel cell integrated with microchannel membrane-based absorption cooling for hydrogen vehicles. Renew. Energy. 178, 560–573 (2021). https://doi.org/10.1016/j.renene.2021.06.098
Yue, M.; Jemei, S.; Zerhouni, N.; Gouriveau, R.: Proton exchange membrane fuel cell system prognostics and decision-making: current status and perspectives. Renew. Energy. 179, 2277–2294 (2021). https://doi.org/10.1016/j.renene.2021.08.045
Trinh, Q.T.; Yang, J.; Lee, J.Y.; Saeys, M.: Computational and experimental study of the volcano behavior of the oxygen reduction activity of PdM@PdPt/C (M = Pt, Ni Co, Fe, and Cr) core–shell electrocatalysts. J. Catal. 291, 26–35 (2012). https://doi.org/10.1016/j.jcat.2012.04.001
Tekalgne, M.A.; Nguyen, K.V.; Nguyen, D.L.T.; Nguyen, V.-H.; Nguyen, T.P.; Vo, D.-V.N.; Trinh, Q.T.; Hasani, A.; Do, H.H.; Lee, T.H.; Jang, H.W.; Le, H.S.; Le, Q.V.; Kim, S.Y.: Hierarchical molybdenum disulfide on carbon nanotube–reduced graphene oxide composite paper as efficient catalysts for hydrogen evolution reaction. J. Alloys Compd. 823, 153897 (2020). https://doi.org/10.1016/j.jallcom.2020.153897
Nihei, M.; Ida, H.; Nibe, T.; Moeljadi, A.M.P.; Trinh, Q.T.; Hirao, H.; Ishizaki, M.; Kurihara, M.; Shiga, T.; Oshio, H.: Ferrihydrite particle encapsulated within a molecular organic cage. J. Am. Chem. Soc. 140, 17753–17759 (2018). https://doi.org/10.1021/jacs.8b10957
Nguyen, V.; Nguyen, T.P.; Le, T.; Vo, D.N.; Nguyen, D.L.; Trinh, Q.T.; Kim, I.T.; Le, Q.V.: Recent advances in two-dimensional transition metal dichalcogenides as photoelectrocatalyst for hydrogen evolution reaction. J. Chem. Technol. Biotechnol. (2020). https://doi.org/10.1002/jctb.6335
Ansari, K.B.; Banerjee, A.; Hassan, S.Z.; Danish, M.; Arman, I.; Khan, P.; Shakeelur Rahman, A.R.; Ahmad, Q.N.; Trinh, Q.T.: Two-dimensional based hybrid materials for photocatalytic conversion of CO2 into hydrocarbon fuels. In: Sadasivuni, K.K.; Kannan, K.; Abdullah, A.M.; Kumar, B. (Eds.) 2D nanomaterials for CO2 conversion into chemicals and fuels, pp. 270–300. The Royal Society of Chemistry, London (2022)
Liu, G.; Narangari, P.R.; Trinh, Q.T.; Tu, W.; Kraft, M.; Tan, H.H.; Jagadish, C.; Choksi, T.S.; Ager, J.W.; Karuturi, S.; Xu, R.: Manipulating intermediates at the Au–TiO2 interface over InP nanopillar array for photoelectrochemical CO2 reduction. ACS Catal. 11, 11416–11428 (2021). https://doi.org/10.1021/acscatal.1c02043
Xu, M.; Xue, H.; Wang, Q.; Jia, L.: Sulfonated poly(arylene ether)s based proton exchange membranes for fuel cells. Int. J. Hydrog. Energy. 46, 31727–31753 (2021). https://doi.org/10.1016/j.ijhydene.2021.07.038
Karimi, M.B.; Mohammadi, F.; Hooshyari, K.: Recent approaches to improve Nafion performance for fuel cell applications: a review. Int. J. Hydrog. Energy. 44, 28919–28938 (2019). https://doi.org/10.1016/j.ijhydene.2019.09.096
Chuy, C.; Basura, V.I.; Simon, E.; Holdcroft, S.; Horsfall, J.; Lovell, K.: V: Electrochemical characterization of ethylenetetrafluoroethylene-g-polystyrenesulfonic acid solid polymer electrolytes. J. Electrochem. Soc. 147, 4453–4458 (2000). https://doi.org/10.1149/1.1394085
Hasani-sadrabadi, M.M.; Mokarram, N.; Azami, M.; Dashtimoghadam, E.; Majedi, F.S.; Jacob, K.I.: Preparation and characterization of nanocomposite polyelectrolyte membranes based on Nafion ionomer and nanocrystalline hydroxyapatite. Polymer (Guildf). 52, 1286–1296 (2011). https://doi.org/10.1016/j.polymer.2010.11.033
Zhang, B.; Cao, Y.; Jiang, S.; Li, Z.; He, G.; Wu, H.: Enhanced proton conductivity of Nafion nanohybrid membrane incorporated with phosphonic acid functionalized graphene oxide at elevated temperature and low humidity. J. Memb. Sci. 518, 243–253 (2016). https://doi.org/10.1016/j.memsci.2016.07.032
Shao, Z.; Joghee, P.; Hsing, I.: Preparation and characterization of hybrid Nafion–silica membrane doped with phosphotungstic acid for high temperature operation of proton exchange membrane fuel cells. J. Memb. Sci. 229, 43–51 (2004). https://doi.org/10.1016/j.memsci.2003.09.014
Lin, C.W.; Lu, Y.S.: Highly ordered graphene oxide paper laminated with a Nafion membrane for direct methanol fuel cells. J. Power Sour. 237, 187–194 (2013). https://doi.org/10.1016/j.jpowsour.2013.03.005
Yang, H.N.; Lee, W.H.; Choi, B.S.; Kim, W.J.: Preparation of Nafion/Pt-containing TiO2/graphene oxide composite membranes for self-humidifying proton exchange membrane fuel cell. J. Memb. Sci. 504, 20–28 (2016). https://doi.org/10.1016/j.memsci.2015.12.021
Tong, X.; Hou, J.; Li, Y.; Li, H.; Wu, W.; Guo, Y.; Liu, Y.; Fu, D.; Huang, X.; Xiong, Z.; Jiang, J.; Qi, L.; Wang, H.; Cai, W.: Application of biochar derived from used cigarette filters in direct carbon solid oxide fuel cell. Int. J. Hydrog. Energy. 47, 22972–22980 (2022). https://doi.org/10.1016/j.ijhydene.2022.05.102
Walkowiak-kulikowska, J.; Wolska, J.; Koroniak, H.: Polymers application in proton exchange membranes for fuel cells (PEMFCs). Phys. Sci. Rev. (2017). https://doi.org/10.1515/psr-2017-0018
Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E.: Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 104, 4587–4612 (2004)
Weiss, R.A.; Sen, A.; Willis, C.L.; Pottick, L.A.: Block copolymer ionomers: 1. Synthesis and physical properties of sulphonated poly(styrene-ethylene/butylene-styrene). Polymer (Guildf). 32, 1867–1874 (1991). https://doi.org/10.1016/0032-3861(91)90378-V
Sannigrahi, A.; Takamuku, S.; Jannasch, P.: Block copolymers combining semi-fluorinated poly(arylene ether) and sulfonated poly(arylene ether sulfone) segments for proton exchange membranes. Int. J. Hydrog. Energy. 39, 15718–15727 (2014). https://doi.org/10.1016/j.ijhydene.2014.07.155
Elabd, Y.A.; Hickner, M.A.: Block copolymers for fuel cells. Macromolecules 44, 1–11 (2011). https://doi.org/10.1021/ma101247c
Lwoya, B.S.; Albert, J.N.L.: Nanostructured block copolymers for proton exchange membrane fuel cells. Energy Environ. Focus. 4, 278–290 (2015). https://doi.org/10.1166/eef.2015.1179
Smitha, B.; Sridhar, S.; Khan, A.A.: Solid polymer electrolyte membranes for fuel cell applications—a review. J. Memb. Sci. 259, 10–26 (2005). https://doi.org/10.1016/j.memsci.2005.01.035
Mistry, M.K.; Roy, N.; Dutta, N.K.; Knott, R.: Inorganic modification of block copolymer for medium temperature proton exchange membrane application. J. Memb. Sci. 351, 168–177 (2010). https://doi.org/10.1016/j.memsci.2010.01.044
Edmondson, C.A.; Fontanella, J.J.; Chung, S.H.; Greenbaum, S.G.; Wnek, G.E.: Complex impedance studies of S-SEBS block polymer proton-conducting membranes. Electrochim. Acta. 46, 1623–1628 (2001). https://doi.org/10.1016/S0013-4686(00)00762-3
Kim, J.; Kim, B.; Jung, B.: Proton conductivities and methanol permeabilities of membranes made from partially sulfonated polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene copolymers. J. Memb. Sci. 207, 129–137 (2002). https://doi.org/10.1016/S0376-7388(02)00138-2
Elabd, Y.A.; Napadensky, E.; Sloan, J.M.; Crawford, D.M.; Walker, C.W.: Triblock copolymer ionomer membranes: part I. Methanol and proton transport. J. Memb. Sci. 217, 227–242 (2003). https://doi.org/10.1016/S0376-7388(03)00127-3
Ghassemi, H.; McGrath, J.E.; Zawodzinski, T.A.: Multiblock sulfonated–fluorinated poly(arylene ether)s for a proton exchange membrane fuel cell. Polymer (Guildf). 47, 4132–4139 (2006). https://doi.org/10.1016/j.polymer.2006.02.038
Shi, Z.; Holdcroft, S.: Synthesis and proton conductivity of partially sulfonated Poly([vinylidene difluoride-co-hexafluoropropylene]-b-styrene) block copolymers. Macromolecules 38, 4193–4201 (2005). https://doi.org/10.1021/ma0477549
Elabd, Y.A.; Napadensky, E.; Walker, C.W.; Winey, K.I.: Transport properties of sulfonated Poly(styrene-b-isobutylene-b-styrene) triblock copolymers at high ion-exchange capacities. Macromolecules 39, 399–407 (2006). https://doi.org/10.1021/ma051958n
Hwang, M.; Nixon, K.; Sun, R.; Willis, C.; Elabd, Y.A.: Sulfonated pentablock terpolymers as membranes and ionomers in hydrogen fuel cells. J. Memb. Sci. 633, 119330 (2021). https://doi.org/10.1016/J.MEMSCI.2021.119330
Huang, F.; Largier, T.D.; Zheng, W.; Cornelius, C.J.: Pentablock copolymer morphology dependent transport and its impact upon film swelling, proton conductivity, hydrogen fuel cell operation, vanadium flow battery function, and electroactive actuator performance. J. Memb. Sci. 545, 1–10 (2018). https://doi.org/10.1016/j.memsci.2017.09.051
Lin, Y.; Yen, C.; Ma, C.M.; Liao, S.; Lee, C.; Hsiao, Y.; Lin, H.: High proton-conducting Nafion ®/–SO3H functionalized mesoporous silica composite membranes. J. Power Sour. 171, 388–395 (2007). https://doi.org/10.1016/j.jpowsour.2007.06.049
Yu, S.; Zuo, X.; Bao, R.; Xu, X.; Wang, J.; Xu, J.: Effect of SiO 2 nanoparticle addition on the characteristics of a new organic–inorganic hybrid membrane. Polymer (Guildf). 50, 553–559 (2009). https://doi.org/10.1016/j.polymer.2008.11.012
Ying, Y.P.; Kamarudin, S.K.; Masdar, M.S.: Silica-related membranes in fuel cell applications : an overview. Int. J. Hydrog. Energy. 43, 16068–16084 (2018). https://doi.org/10.1016/j.ijhydene.2018.06.171
Su, Y.H.; Liu, Y.L.; Sun, Y.M.; Lai, J.Y.; Wang, D.M.; Gao, Y.; Liu, B.; Guiver, M.D.: Proton exchange membranes modified with sulfonated silica nanoparticles for direct methanol fuel cells. J. Memb. Sci. 296, 21–28 (2007). https://doi.org/10.1016/j.memsci.2007.03.007
Pal, S.; Mondal, R.; Chatterjee, U.: Sulfonated polyvinylidene fluoride and functional copolymer based blend proton exchange membrane for fuel cell application and studies on methanol crossover. Renew. Energy. 170, 974–984 (2021). https://doi.org/10.1016/j.renene.2021.02.046
Yagizatli, Y.; Sahin, A.; Ar, I.: Effect of thermal crosslinking process on membrane structure and PEM fuel cell applications performed with SPEEK-PVA blend membranes. Int. J. Hydrog. Energy. (2022). https://doi.org/10.1016/j.ijhydene.2022.04.183
Alnaqbi, H.; Sayed, E.T.; Al-Asheh, S.; Bahaa, A.; Alawadhi, H.; Abdelkareem, M.A.: Current progression in graphene-based membranes for low temperature fuel cells. Int. J. Hydrog. Energy (2022). https://doi.org/10.1016/j.ijhydene.2022.03.255
Teixeira, F.C.; de Sá, A.I.; Teixeira, A.P.S.; Rangel, C.M.: Enhanced proton conductivity of Nafion-azolebisphosphonate membranes for PEM fuel cells. New J. Chem. 43, 15249–15257 (2019). https://doi.org/10.1039/C9NJ03405F
Herz, H.G.; Kreuer, K.D.; Maier, J.; Scharfenberger, G.; Schuster, M.F.H.; Meyer, W.H.: New fully polymeric proton solvents with high proton mobility. Electrochim. Acta. 48, 2165–2171 (2003). https://doi.org/10.1016/S0013-4686(03)00200-7
Duong, P.H.H.; Chung, T.-S.; Wei, S.; Irish, L.: Highly permeable double-skinned forward osmosis membranes for anti-fouling in the emulsified oil–water separation process. Environ. Sci. Technol. 48, 4537–4545 (2014). https://doi.org/10.1021/es405644u
Shi, G.M.; Zuo, J.; Tang, S.H.; Wei, S.; Chung, T.S.: Layer-by-layer (LbL) polyelectrolyte membrane with NexarTM polymer as a polyanion for pervaporation dehydration of ethanol. Sep. Purif. Technol. 140, 13–22 (2015). https://doi.org/10.1016/j.seppur.2014.11.008
Sivsankaran, A.; Sangeetha, D.; Young-ho, A.: Nanocomposite membranes based on sulfonated polystyrene ethylene butylene polystyrene (SSEBS) and sulfonated SiO2 for microbial fuel cell application. Chem. Eng. J. (2015). https://doi.org/10.1016/j.cej.2015.12.095
Sivasankaran, A.; Sangeetha, D.: Influence of sulfonated SiO2 in sulfonated polyether ether ketone nanocomposite membrane in microbial fuel cell. Fuel 159, 689–696 (2015)
Ke, C.-C.; Li, X.-J.; Shen, Q.; Qu, S.-G.; Shao, Z.-G.; Yi, B.-L.: Investigation on sulfuric acid sulfonation of in-situ sol–gel derived Nafion/SiO2 composite membrane. Int. J. Hydrog. Energy. 36, 3606–3613 (2011). https://doi.org/10.1016/j.ijhydene.2010.12.030
Reddy, K.R.; Lee, K.-P.; Gopalan, A.I.; Kang, H.-D.: Organosilane modified magnetite nanoparticles/poly(aniline-co-o/m-aminobenzenesulfonic acid) composites: Synthesis and characterization. React. Funct. Polym. 67, 943–954 (2007). https://doi.org/10.1016/j.reactfunctpolym.2007.05.023
Yu, D.M.; Yoon, Y.J.; Kim, T.; Lee, J.Y.; Hong, Y.T.: Sulfonated poly (arylene ether sulfone)/ sulfonated zeolite composite membrane for high temperature proton exchange membrane fuel cells. Solid State Ionics 233, 55–61 (2013). https://doi.org/10.1016/j.ssi.2012.12.006
Park, C.H.; Lee, C.H.; Guiver, M.D.; Lee, Y.M.: Sulfonated hydrocarbon membranes for medium-temperature and low-humidity proton exchange membrane fuel cells ( PEMFCs ). Prog. Polym. Sci. 36, 1443–1498 (2011). https://doi.org/10.1016/j.progpolymsci.2011.06.001
Chien, H.C.; Tsai, L.D.; Huang, C.P.; Kang, C.Y.; Lin, J.N.; Chang, F.C.: Sulfonated graphene oxide/Nafion composite membranes for high-performance direct methanol fuel cells. Int. J. Hydrog. Energy. 38, 13792–13801 (2013). https://doi.org/10.1016/j.ijhydene.2013.08.036
Liu, Y.; Wang, J.; Zhang, H.; Ma, C.; Liu, J.; Cao, S.; Zhang, X.: Enhancement of proton conductivity of chitosan membrane enabled by sulfonated graphene oxide under both hydrated and anhydrous conditions. J. Power Sour. 269, 898–911 (2014). https://doi.org/10.1016/j.jpowsour.2014.07.075
Singha, S.; Koyilapu, R.; Dana, K.; Jana, T.: Polybenzimidazole-clay nanocomposite membrane for PEM fuel cell: effect of organomodifier structure. Polymer (Guildf). 167, 13–20 (2019). https://doi.org/10.1016/j.polymer.2019.01.066
Shin, D.W.; Guiver, M.D.; Lee, Y.M.: Hydrocarbon-based polymer electrolyte membranes: importance of morphology on Ion transport and membrane stability. Chem. Rev. 117, 4759–4805 (2017). https://doi.org/10.1021/acs.chemrev.6b00586
Seol, J.; Won, J.; Yoon, K.; Hong, Y.T.; Lee, S.: SiO2 ceramic nanoporous substrate-reinforced sulfonated poly (arylene ether sulfone) composite membranes for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 37, 6189–6198 (2012). https://doi.org/10.1016/j.ijhydene.2011.06.085
Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M.: Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrog. Energy 35, 9349–9384 (2010). https://doi.org/10.1016/j.ijhydene.2010.05.017
Thiam, H.S.; Daud, W.R.W.; Kamarudin, S.K.; Mohammad, A.B.; Kadhum, A.A.H.; Loh, K.S.; Majlan, E.H.: Overview on nanostructured membrane in fuel cell applications. Int. J. Hydrogen Energy. 36, 3187–3205 (2011). https://doi.org/10.1016/j.ijhydene.2010.11.062
Ghosh, S.; Maity, S.; Jana, T.: Polybenzimidazole/silica nanocomposites : organic-inorganic hybrid membranes for PEM fuel cell. J. Mater. Chem. 21, 14897–14906 (2011). https://doi.org/10.1039/c1jm12169c
Parthiban, V.; Akula, S.; Peera, S.G.; Islam, N.; Sahu, A.K.: Proton conducting nafion-sulfonated graphene hybrid membranes for direct methanol fuel cells with reduced methanol crossover. Energy Fuels 30, 725–734 (2016). https://doi.org/10.1021/acs.energyfuels.5b02194
Novikova, S.A.; Yurkov, G.Y.; Yaroslavtsev, A.B.: Synthesis of copper and silver nanoparticles in MF-4SC and sulfonated poly(ether ether ketone) membranes and transport properties of the composites. Inorg. Mater. 46, 793–798 (2010). https://doi.org/10.1134/S0020168510070198
Vinothkannan, M.; Kim, A.R.; Kumar, G.G.; Yoon, J.; Yoo, D.J.: Toward improved mechanical strength, oxidative stability and proton conductivity of an aligned quadratic hybrid (SPEEK/FPAPB/Fe3O4-FGO) membrane for application in high temperature and low humidity fuel cells. RSC Adv. 7, 39034–39048 (2017). https://doi.org/10.1039/c7ra07063b
Ansari, K.B.; Gaikar, V.G.; Trinh, Q.T.; Khan, M.S.; Banerjee, A.; Kanchan, D.R.; Mesfer, M.K.A.; Danish, M.: Carbon dioxide capture over amine functionalized styrene divinylbenzene copolymer: an experimental batch and continuous studies. J. Environ. Chem. Eng. 10, 106910 (2022). https://doi.org/10.1016/j.jece.2021.106910
Amaniampong, P.N.; Trinh, Q.T.; Varghese, J.J.; Behling, R.; Valange, S.; Mushrif, S.H.; Jérôme, F.: Unraveling the mechanism of the oxidation of glycerol to dicarboxylic acids over a sonochemically synthesized copper oxide catalyst. Green Chem. 20, 2730–2741 (2018). https://doi.org/10.1039/C8GC00961A
Amaniampong, P.N.; Trinh, Q.T.; Wang, B.; Borgna, A.; Yang, Y.; Mushrif, S.H.: Biomass oxidation: formyl C–H bond activation by the surface lattice oxygen of regenerative CuO nanoleaves. Angew. Chemie Int. Ed. 54, 8928–8933 (2015). https://doi.org/10.1002/anie.201503916
Amaniampong, P.N.; Trinh, Q.T.; De Oliveira Vigier, K.; Dao, D.Q.; Tran, N.H.; Wang, Y.; Sherburne, M.P.; Jérôme, F.: Synergistic effect of high-frequency ultrasound with cupric oxide catalyst resulting in a selectivity switch in glucose oxidation under argon. J. Am. Chem. Soc. 141, 14772–14779 (2019). https://doi.org/10.1021/jacs.9b06824
Paul, R.; Shit, S.C.; Fovanna, T.; Ferri, D.; Srinivasa Rao, B.; Gunasooriya, G.T.K.K.; Dao, D.Q.; Le, Q.V.; Shown, I.; Sherburne, M.P.; Trinh, Q.T.; Mondal, J.: Realizing catalytic acetophenone hydrodeoxygenation with palladium-equipped porous organic polymers. ACS Appl. Mater. Interfaces 12, 50550–50565 (2020). https://doi.org/10.1021/acsami.0c16680
Paolo, F.: Biomolecular electronics. Elsevier, Hoboken (2014)
Massimiliano, L.F.: Solid oxide-based electrochemical devices. Elsevier, Hoboken (2020)
Ansari, M.Y.; Rizvi, S.J.A.; Inamuddin: Preparation and properties of novel sulfonated pentablock copolymer sPBC membrane for PEM fuel cell. In: Yadav, S.; Singh, D.B.; Arora, P.K.; Kumar, H. (Eds.) Proceedings of international conference in mechanical and energy technology: ICMET 2019 India, pp. 613–621. Springer, Singapore (2020)
Acknowledgements
The authors would like to acknowledge University Sophisticated Instrumentation Facility (USIF) and Department of Chemistry and Department of Physics, Aligarh Muslim University, Aligarh, India.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansari, M.Y., Ansari, K.B., Inamuddin et al. Silica and Sulfonated Silica Functionalized Nexar Nanocomposite Membranes for Application in Proton Exchange Membrane Fuel Cell. Arab J Sci Eng 48, 16187–16199 (2023). https://doi.org/10.1007/s13369-023-08085-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-08085-0