Abstract
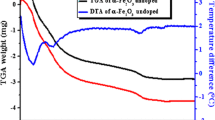
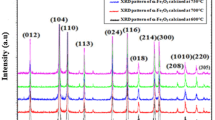
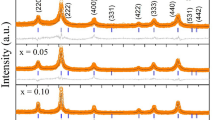
In this paper, hematite (α-Fe2O3) nanoparticles were prepared at two different temperatures (500 and 600 °C) by one-step direct thermal decomposition of Fe(NO3)2·6H2O at the presence of urea (1:1 weight ratio). The products were named as Fe-1 and Fe-2 and characterized by X-ray diffraction (XRD), vibrating sample magnetometer (VSM) and transmission electron microscopy (TEM). The photocatalytic activities of the products were studied by degradation of methyl green (MG) dye in aqueous solution at room temperature. According to the XRD results, the average size of crystallites Fe-2 is 110 nm. The applied method is fast, facile and does not use any solvents. The magnetic properties show that the saturation magnetization of Fe-2 (≈ 0.9 emu/g) is higher than that of Fe-1 (≈ 0.6 emu/g) which confirms the higher purity of α-phase of Fe-2. In addition, the products were used for the degradation of MG from aqueous solution. The influence of various parameters such as contact time and mass of the α-Fe2O3 nanoparticles has been studied. The photocatalytic results predict that the as-prepared hematite (α-Fe2O3) nanoparticles have potential to be used as an efficient degradation agent of MG and other cationic dyes.







Similar content being viewed by others
References
Xu, D.; Liu, J.; Ma, T.; Zhao, X.; Ma, H.; Li, J.: Coupling of sponge fillers and two-zone clarifiers for granular sludge in an integrated oxidation ditch. Environ. Technol. Innov. 26, 102264 (2022)
Guo, C.; Zhang, Z.; Wu, Y.; Wang, Y.; Ma, G.; Shi, J.; Zhao, Y.: Synergic realization of electrical insulation and mechanical strength in liquid nitrogen for high-temperature superconducting tapes with ultra-thin acrylic resin coating. Superconduct. Sci. Technol. 35, 075014 (2022)
Hu, M.; Wang, Y.; Yan, Z.; Zhao, G.; Zhao, Y.; Xia, L.; Zhuang, X.: Hierarchical dual-nanonet of polymer nanofibers and supramolecular nanofibrils for air filtration with a high filtration efficiency, low air resistance and high moisture permeation. J. Mater. Chem. A. Mater. Energy Sustain. 9, 14093–14100 (2021)
Hou, C.; Yin, M.; Lan, P.; Wang, H.; Nie, H.; Ji, X.: Recent progress in the research of Angelica sinensis (Oliv.) Diels polysaccharides: extraction, purification, structure and bioactivities. Chem. Biol. Technol. Agricul. 8, 1–14 (2021)
Xiong, Q.; Chen, Z.; Huang, J.; Zhang, M.; Song, H.; Hou, X.; Feng, Z.: Preparation, structure and mechanical properties of Sialon ceramics by transition metal-catalyzed nitriding reaction. Rare Met. 39, 589–596 (2020)
Fu, Y.; Chen, H.; Guo, R.; Huang, Y.; Toroghinejad, M.R.: Extraordinary strength-ductility in gradient amorphous structured Zr-based alloy. J. All. Comp. 888, 161507 (2021)
Wang, G.; Liu, D.; Fan, S.; Li, Z.; Su, J.: High-k erbium oxide film prepared by sol-gel method for low-voltage thin-film transistor. Nanotechnol. 32, 215202 (2021)
Yan, W.; Cao, M.; Fan, S.; Liu, X.; Liu, T.; Li, H.; Su, J.: Multi-yolk ZnSe/2(CoSe2)@NC heterostructures confined in N-doped carbon shell for high-efficient sodium-ion storage. Compos. B Eng. 213, 108732 (2021)
Khalaji, A.D.; Pazhand, Z.; Kiani, K.; Machek, P.; Jarosova, M.; Mazandarani, R.: CuO nanoparticles: preparation, characterization, optical properties, and antibacterial activities. J. Mater. Sci: Mater. Electron. 31, 11949–11954 (2020)
Bai, Z.; Zhang, X.; Zhang, Y.; Guo, C.; Tang, B.: Facile synthesis of mesoporous Mn3O4 nanorods as a promising anode material for high performance lithium-ion batteries. J. Mater. Chem. A 2, 16755–16760 (2014)
Khalaji, A.D.; Jarosova, M.; Machek, P.; Chen, K.; Xue, D.: Facile synthesis, characterization and electrochemical performance of nickel oxide nanoparticles prepared by thermal decomposition. Scripta Mater. 181, 53–57 (2020)
Khalaji, A.D.; Jarosova, M.; Machek, P.; Chen, K.; Xue, D.: Co3O4 Nanoparticles: synthesis, characterization and its application as performing anode in Li-Ion batteries. J. Nanostruct. 10, 607–612 (2020)
Pushpanathan, V.; Suresh Kumar, D.: A novel zinc(II) macrocycle-based synthesis of pure ZnO nanoparticles. J. Nanostruct. Chem. 4, 95–101 (2014)
Yan, W.; Liang, K.; Chi, Z.; Liu, T.; Cao, M.; Fan, S.; Su, J.: Litchi-like structured MnCo2S4@C as a high capacity and long-cycling time anode for lithium-ion batteries. Electrochim. Acta 376, 138035 (2021)
Lu, T.; Yan, W.; Feng, G.; Luo, X.; Hu, Y.; Guo, J.; Ding, S.: Singlet oxygen-promoted one-pot synthesis of highly ordered mesoporous silica materials via the radical route. Green Chem. (2022). https://doi.org/10.1039/D2GC00869F
Zhou, J.; He, Y.; Shen, J.; Essa, F.A.; Yu, J.: Ni/Ni3Al interface-dominated nanoindentation deformation and pop-in events. Nanotechnol. 33, 105703 (2021)
Zhang, Y.; Nan, L.I.; Li, C.; Huang, C.; Ali, H.M.; Xu, X.; Mao, C.; Ding, W.; Cui, X.; Yang, M.; Yu, T.; Jamil, M.; Gupta, M.K.; Jia, D.; Said, Z.: Nano-enhanced biolubricant in sustainable manufacturing: from processability to mechanisms. Friction 10, 803–841 (2022)
Liu, M.; Li, C.; Zhang, Y.; An, Q.; Yang, M.; Gao, T.; Mao, C.; Liu, B.; Cao, H.; Xu, X.; Said, Z.; Debnath, S.; Jamil, M.; Ali, H.M.; Sharma, S.: Cryogenic minimum quantity lubrication machining: from mechanism to application. Front. Mech. Eng. 16, 649–697 (2021)
Sharma, S.; Dhiman, N.; Kumar, A.; Singh, M.; Dhiman, P.: Effect of synthesis on optical and magnetic properties of Fe2O3 nanoparticles. Integ. Ferroelect. 204, 38–46 (2020)
. Rahman, G., Najaf, Z., ul Haq Ali Shah, A., Mian, S.A.: Investigation of the structural, optical and photoelectrochemical properties of α-Fe2O3 nanorods synthesized via a facile chemical bath deposition. Optik 200:163454 (2020).
Al-Douri, Y.; Amrane, N.; Johan, M.R.: Annealing temperature effect on structural and optical investigations of Fe2O3 nanostructure. J. Mater. Res. Technol. 8, 2164–2169 (2019)
Mazrouaa, A.M.; Mohamed, M.G.; Fekry, M.: Physical and magnetic properties of iron oxide nanoparticles with a different molar ratio of ferrous and ferric. Egypt. J. Petrol. 28, 165–171 (2019)
Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Ammar, A.; Gadri, A.: Synthesis, photoluminescence and magnetic properties of iron oxide (Fe2O3) nanoparticles through precipitation or hydrothermal methods. Phys. E. 101, 212–219 (2018)
Jamzad, M.; Karimi Bidkorpeh, M.: Green synthesis of iron oxide nanoparticles by the aqueous extract of Laurus nobilis L. leaves and evaluation of the antimicrobial activity. J. Nanostruct. Chem. 10, 193–201 (2020)
Raham, G.; Joo, O.S.: Facile preparation of nanostructured α-Fe2O3 thin films with enhanced photoelectrochemical water splitting activity. J. Mater. Chem. A 1, 5554–5561 (2013)
Li, Z.; Mao, Y.; Tian, Q.; Zhang, W.; Yang, L.: Extremely facile preparation of high-performance Fe2O3 anode for lithium-ion batteries. J. All. Compd. 784, 125–133 (2019)
Al-Atta, A.; Sher, F.; Hazafa, A.; Zafar, A.; Iqbal, H.M.N.; Karahmet, E.; Lester, E.: Supercritical water oxidation of phenol and process enhancement with in situ formed Fe2O3 nano catalyst. Environ. Sci. Pull. Res. (2021). https://doi.org/10.1007/s11356-021-16390-0
Fu, C.; Mahadevegowda, A.; Grant, P.S.: Production of hollow and porous Fe2O3 from industrial mill scale and its potential for large-scale electrochemical energy storage applications. J. Mater. Chem. A 4, 2597–2604 (2016)
Wang, Y.; Mao, P.; Yan, F.; Gao, C.; Liu, Y.; Ding, J.; Wu, W.; Liu, Y.: Flower-like Fe2O3/reduced graphene oxide composite for electrochemical energy storage. Synth. Met. 222, 198–204 (2016)
Hung, C.M.; Hoa, N.D.; Duy, N.V.; Toan, N.V.; Le, D.T.T.; Hieu, N.V.: Synthesis and gas-sensing characteristics of α-Fe2O3 hollow balls. J. Sci: Adv. Mater. Dev. 1, 45–50 (2016)
Mirzaei, A.; Hashemi, B.; Janghorban, K.: α-Fe2O3 based nanomaterials as gas sensors. J. Mater. Sci: Mater. Electron. 27, 3109–3144 (2016)
Shahriai, T.; Mehrdadi, N.; Tahmasebi, M.: Study of cadmium and nickel removal from battery industry wastewater by Fe2O3 nanoparticles. Pollution 5, 515–524 (2019)
Tamez, C.; Hernandez, R.; Parsons, J.G.: Removal of Cu(II) and Pb(II) from aqueous solution using engineered iron oxide nanoparticles. Microchem. J. 125, 97–104 (2016)
Jerin, V.M.; Remya, R.; Mariyam, T.; Varkey, J.T.: Investigation of the removal of toxic chromium ion from waste water using Fe2O3 nanoparticles. Mater. Today: Proceed. 9, 27–31 (2019)
Gandha, K.; Mohapatra, J.; Kabir Hossain, M.; Elkins, K.; Poudyal, N.; Rajeshwar, K.; Liu, J.P.: Mesoporous iron oxide nanowires: synthesis, magnetic and photocatalytic properties. RSC Adv. 6, 90537–90546 (2016)
Ye, C.; Hu, K.; Niu, Z.; Lu, Y.; Zhang, L.; Yan, K.: Controllable synthesis of rhombohedral α-Fe2O3 efficient for photocatalytic degradation of bisphenol A. J. Water Process. 27, 205–210 (2019)
Kusior, A.; Michalec, K.; Jelen, P.; Radecka, M.: Shaped Fe2O3 nanoparticles – synthesis and enhanced photocatalytic degradation towards RhB. Appl. Surf. Sci. 476, 342–352 (2019)
Taghavi Fardood, S.; Moradnia, F.; Moradi, S.; Forootan, R.; Yekke Zare, F.; Heidari, M.: Eco-friendly synthesis and characterization of α-Fe2O3 nanoparticles and study of their photocatalytic activity for degradation of congo red dye. Nanochem. Res. 4, 140–147 (2019)
Wang, J.; Shao, X.; Zhang, Q.; Tian, G.; Ji, X.; Bao, W.: Preparation of mesoporous magnetic Fe2O3 nanoparticles and its application for organic dyes removal. J. Mol. Liq. 248, 13–18 (2017)
Dissanayale, D.M.S.N.; Mantilaka, M.M.M.G.P.G.; Palihawadana, T.C.; Chandrakumara, G.T.D.; De Silva, R.T.; Pitawala, H.M.T.G.A.; Nalin de Silva, K.M.; Amaratunga, G.A.H.: Facile and low-cost synthesis of pure hematite (α-Fe2O3) nanoparticles from naturally occurring laterites and their superior adsorption capability towards acid-dyes. RSC Adv. 9, 21249–21257 (2019)
Debnath, A.; Deb, K.; Chattopadhyay, K.K.; Saha, B.: Methyl orange adsorption onto simple chemical route synthesized crystalline α-Fe2O3 nanoparticles: kinetic, equilibrium isotherm, and neural network modeling. Desal. Water Treat. 57, 13549–13560 (2016)
Cao, Y.; Alamri, S.; Rajhi, A.A.; Anqi, A.E.; Khalaji, A.D.: New chitosan Schiff base and its nanocomposite: removal of methyl green from aqueous solution and its antibacterial activities. Int. J. Biol. Macromol. 192, 1–6 (2021)
Foroughnia, A.; Khalaji, A.D.; Kolvari, E.; Koukabi, N.: Synthesis of new chitosan Schiff base and its Fe2O3 nanocomposite: evaluation of methyl orange removal and antibacterial activity. Int. J. Biol. Macromol. 177, 83–91 (2021)
Cinar, S.; Kaynar, U.H.; Aydemir, T.; Kaynar, S.C.; Ayvacikli, M.: An efficient removal of RB5 from aqueous solution by adsorption onto nano-ZnO/chitosan composite beads. Int. J. Biol. Macromol. 96, 459–465 (2017)
Farghali, A.A.; Bahgat, M.; El Rouby, W.M.A.; Khedr, M.H.: Preparation, decoration and characterization of graphene sheets for methyl green adsorption. J. Alloys Compd. 555, 193–200 (2013)
Bahgat, M.; Farghali, A.A.; El Rouby, W.; Khedr, M.; Mohassab-Ahmed, M.Y.: Adsorption of methyl green dye onto multi-walled carbon nanotubes decorated with Ni nanoferrite. Appl. Nanosci. 3, 251–261 (2013)
Bashandeh, Z.; Khalaji, A.D.: An efficient removal of methyl green dye by adsorption onto new modified chitosan Schiff base. Asian J. Nanosci. Mater. 4, 274–281 (2021)
Rida, K.; Chaibeddra, K.; Cheraitia, K.: Adsorption of cationic dye methyl green from aqueous solution onto activated carbon prepared from BrachychitonPopulneus fruit shell. Ind. J. Chem. Technol. 27, 51–59 (2020)
Sharma, P.; Saikia, B.K.; Dasa, M.R.: Removal of methyl green dye molecule from aqueous system using reduced graphene oxide as an efficient adsorbent: kinetics, isotherm and thermodynamic parameters Ponchami. Coll. Surf. A: Physicochem. Eng. Aspects 457, 125–133 (2014)
Bashandeh, Z.; Khalaji, A.D.: Effective removal of methyl green from aqueous solution using epichlorohydrine cross-linked chitosan. Adv. J. Chem. A 4, 270–277 (2021)
Abbas, M.; Aksil, T.; Trari, M.: Removal of toxic methyl green (MG) in aqueous solutions by apricot stone activated carbon – equilibrium and isotherms modeling. Desalin. Water Treat. 125, 93–101 (2018)
Mahmoud, N.M.R.; El-Moselhy, M.M.; Alkhaldi, M.A.: Remediation of methyl green dye from aqueous solution via adsorption and degradation using silica gel modified with hydrated zinc oxide catalyst. Desalin. and Water Treat. 158, 385–397 (2019)
Ansari, M.J.; Jasim, S.A.; Bokov, D.O.; Thangavelu, L.; Yasin, G.; Khalaji, A.D.: Preparation of new bio-based chitosan/Fe2O3/NiFe2O4 as an efficient removal of methyl green from aqueous solution. Int. J. Biol. Macromol. 198, 128–134 (2022)
Hadjltaief, H.B.; Zina, M.B.; Galvez, M.E.; Da Costa, P.: Photocatalytic degradation of methyl green dye in aqueous solution over natural clay-supported ZnO–TiO2 catalysts. J. Photochem. Photobiol. A: Chem. 315, 25–33 (2016)
Mai, F.D.; Chen, C.C.; Chen, J.L.; Liu, S.C.: Photodegradation of methyl green using visible irradiation in ZnO suspensions determination of the reaction pathway and identification of intermediates by a high-performance liquid chromatography–photodiode array-electrospray ionization-mass spectrometry method. J. Chromatogr. A. 1189, 355–365 (2008)
Nezamzadeh-Ejhieh, A.; Shams-Ghahfarokhi, Z.: Photodegradation of methyl green by nickel-dimethylglyoxime/ZSM-5 zeolite as a heterogeneous catalyst. J. Chem. Article ID 104093, 11 pages (2013).
Kumar, A.; Pandey, G.: The photocatalytic degradation of methyl green in presence of visible light with photoactive Ni0.1:La0.05:TiO2 nanocomposites. IOSR J. Appl. Chem. 10, 31–44 (2017)
Liu, J.; Yang, H.; Xue, X.: Preparation of different shaped α-Fe2O3 nanoparticles with large particle of iron oxide red. CrystEngComm 21, 1097–1101 (2019)
Khalaji, A.D.; Mousavia, S.M.; Jarosova, M.; Machek, P.: The effect of calcination temperature on the morphology, size and the crystalline phase of the as-synthesized products by direct calcination of FeSO4 at the presence of benzoic acid. J. Surf. Invest: X-ray Synch. Neut. Tech. 14, 1191–1194 (2020)
Khalaji, A.D.; Ghorbani, M.: Thermal studies of iron (II) Schiff base complexes: new precursors for preparation of α-Fe2O3 nanoparticles via solid-state thermal decomposition. Chem. Method. 4, 532–542 (2020)
Ahmadzadeh, M.; Romero, C.; McCloy, J.: Magnetic analysis of commercial hematite, magnetite, and their mixtures. AIP Adv. 8, 056807 (2021)
Ahmad, T.; Iqbal, J.; Bustam, M.A.; Zulfiqar, M.; Muhammad, N.; Al Hajeri, B.M.; Irfan, M.; Anwaar Asghar, H.M.; Ullah, S.: Phytosynthesis of cerium oxide nanoparticles and investigation of their photocatalytic potential for degradation of phenol under visible light. J. Mol. Struct. 1217, 128292 (2020)
Majumder, D.; Chakraborty, I.; Mandal, K.; Roy, S.: Facet-dependent photodegradation of methylene blue using pristine CeO2 nanostructures. ACS Omega 4, 4243–4251 (2019)
Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Ammar, S.; Gadri, A.: Nanocrystalline NixCo(0.5-x)Zn0.5Fe2O4 ferrite: fabrication through co-precipitation route with enhanced structural, magnetic and photocatalytic activity. J. Mater. Sci: Mater. Electron. 29, 7333–7344 (2018)
Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Ammar, S.; Gadri, A.: Photocatalytic degradation of methyl orange dye by NiFe2O4 nanoparticles under visible irradiation: effect of varying the synthesis temperature. J. Mater. Sci: Mater. Electron. 29, 7057–7067 (2018)
Yuvaraja, G.; Chen, D.Y.; Pathak, J.L.; Long, J.; Subbaiah, M.V.; Wen, J.C.; Pan, C.L.: Preparation of novel aminated chitosan schiff’s base derivative for the removal of methyl orange dye from aqueous environment and its biological applications. Int. J. Biol. Macromol. 146, 1100–1110 (2020)
Parshi, N.; Pan, D.; Dhavle, V.; Jana, B.; Maity, S.; Ganguly, J.: Fabrication of lightweight and reusable salicylaldehyde functionalized chitosan as adsorbent for dye removal and its mechanism. Int. J. Biol. Macromol. 141, 626–635 (2019)
Zhai, L.; Bai, Z.; Zhu, Y.; Wang, B.; Luo, W.: Fabrication of chitosan microspheres for efficient adsorption of methyl orange. Chin. J. Chem. Eng. 26, 657–666 (2018)
Moghaddam, A.Z.; Ghiamati, E.; Pourashuri, A.; Allahresani, A.: Modified nickel ferrite nanocomposite/functionalized chitosan as a novel adsorbent for the removal of acidic dyes. Int. J. Biol. Macromol. 120, 1714–1725 (2018)
Anitha, T.; Senthil Kumar, P.; Sathish Kumar, K.: Synthesis of nano-sized chitosan blended polyvinyl alcohol for the removal of Eosin Yellow dye from aqueous solution. J. Water Process Eng. 13, 127–136 (2016)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brontowiyono, W., AbdulHussein, W.A., Smaisim, G.F. et al. Annealing Temperature Effect on Structural, Magnetic Properties and Methyl Green Degradation of Fe2O3 Nanostructures. Arab J Sci Eng 48, 375–382 (2023). https://doi.org/10.1007/s13369-022-07118-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-07118-4