Abstract
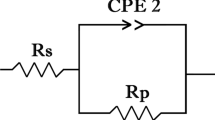
Electrochemical deposition of Ni metal matrix composite coatings is widely used in various applications to improve corrosion resistance and hardness of the materials. In this study, the Ni-Cu and Ni-Cu/CeO2 metal matrix coatings were fabricated via electrodeposition on SS 304 substrates. The cyclic voltammetry measurements suggest that the addition of CeO2 particles into the electrolyte shifted hydrogen evolution reaction to more negative potentials and slightly accelerated the deposition of Ni ions. The compositional analyses also suggested a higher Ni fraction for the deposit with CeO2 containing electrolyte. The structural characterization through XRD measurements showed the presence of Ni-Cu solid solutions for all coatings and the presence of CeO2 for the ceramic reinforced layer. The crystal size of the Ni-Cu solid solutions ranged between 10 and 20 nm. It was observed that the morphology of the coatings was cauliflower and the size of the cauliflowers changed to a bigger size and turned into compacted layer with the addition of CeO2 into electrolyte. Deposits without CeO2 showed smaller and more localized separated cauliflowers, whereas coatings with CeO2 showed denser layer. The hardness of the samples increased from 317 ± 19 to 475 ± 18 HV and the corrosion current density decreased from 48.8 to 36.5 µA/cm2 with the addition of CeO2 suggesting an improved performance for Ni-Cu/CeO2 metal matrix composite coating. The Nyquist and Bode plots supported the findings in the potentiodynamic polarization tests.











Similar content being viewed by others
References
ASM, Introduction to surface engineering for corrosion and wear resistance. (2001)
Tahri, W.; Hu, X.; Shi, C.; Zhang, Z.: Review on corrosion of steel reinforcement in alkali-activated concretes in chloride-containing environments. Constr. Build. Mater. 293, 123484 (2021). https://doi.org/10.1016/j.conbuildmat.2021.123484
Zaffora, A.; Franco, F.D.; Santamaria, M.: Electrochemistry Corrosion of stainless steel in food and pharmaceutical industry. Curr. Opin. Electrochem. 29, 100760 (2021). https://doi.org/10.1016/j.coelec.2021.100760
Attarzadeh, N.; Molaei, M.; Babaei, K.; Fattah-alhosseini, A.: New promising ceramic coatings for corrosion and wear protection of steels: a review. Surf. Interfaces. 23, 100997 (2021). https://doi.org/10.1016/j.surfin.2021.100997
Khaled, K.; Berardi, U.: Current and future coating technologies for architectural glazing applications. Energy Build. 244, 111022 (2021). https://doi.org/10.1016/j.enbuild.2021.111022
You, Q.; Xiong, J.; Guo, Z.; Huo, Y.; Liang, L.; Yang, L.: Study on coating performance of CVD coated cermet tools for 4340 steel cutting. Int. J. Refract. Met. Hard Mater. 98, 105554 (2021). https://doi.org/10.1016/j.ijrmhm.2021.105554
Mohammed, I.K.; Havaldar, S.S.; Hiriyannaiah, A.: Composition optimization for NiAl + Al 2 O 3 + CeO composite coating on bearing steel by air plasma spray. Mater. Today Proc (2021). https://doi.org/10.1016/j.matpr.2021.01.139
Reddy, M.; Prasad, C.D.; Patil, P.; Ramesh, M.R.; Rao, N.: Hot corrosion behavior of plasma-sprayed NiCrAlY / TiO 2 and NiCrAlY / Cr 2 O 3 / YSZ cermets coatings on alloy steel. Surf. Interfaces. 22, 100810 (2021). https://doi.org/10.1016/j.surfin.2020.100810
Carvalho, P.; Jiang, Y.; Serpe, M.J.: Portable point-of-care diagnostic devices. Anal. Methods. (2016). https://doi.org/10.1039/c6ay02158a
Muzammal, M.; Ma, H.; Huang, M.; Gao, Z.; Cao, J.; Wang, C.; Dong, C.; Wang, Y.; Kunwar, A.: Fabrication of cerium myristate coating for a mechanochemically robust modifier-free superwettability system to enhance the corrosion resistance on 316L steel by one-step electrodeposition. Surf. Coat. Technol. 398, 125970 (2020). https://doi.org/10.1016/j.surfcoat.2020.125970
Walsh, F.C.; Wang, S.; Zhou, N.: The electrodeposition of composite coatings : Diversity, applications and challenges. Curr. Opin. Electrochem. 20, 8–19 (2020). https://doi.org/10.1016/j.coelec.2020.01.011
Costa, J.M.; Florêncio, A.; Neto, D.A.: Ultrasound-assisted electrodeposition and synthesis of alloys and composite materials : A review. Ultrason. - Sonochemistry. 68, 105193 (2020). https://doi.org/10.1016/j.ultsonch.2020.105193
Torkamani, A.D.; Velashjerdi, M.; Abbas, A.; Bolourchi, M.; Maji, P.: Electrodeposition of nickel matrix composite coatings via various boride particles: a review. J. Compos. Compd. 3, 106–113 (2021)
Protsenko, V.S.; Bogdanov, D.A.; Korniy, S.A.; Kityk, A.A.: Application of a deep eutectic solvent to prepare nanocrystalline Ni and Ni / TiO 2 coatings as electrocatalysts for the hydrogen evolution reaction. Int. J. Hydrogen Energy. 44, 24604–24616 (2019). https://doi.org/10.1016/j.ijhydene.2019.07.188
Ma, C.Y.; Zhao, D.Q.; Xia, F.F.; Xia, H.; Williams, T.; Xing, H.Y.: Ultrasonic-assisted electrodeposition of Ni-Al 2 O 3 nanocomposites at various ultrasonic powers. Ceram. Int. 46, 6115–6123 (2020). https://doi.org/10.1016/j.ceramint.2019.11.075
Ji, R.; Han, K.; Jin, H.; Li, X.; Liu, Y.; Liu, S.; Dong, T.; Cai, B.; Cheng, W.: Preparation of Ni-SiC nano-composite coating by rotating magnetic fi eld- assisted electrodeposition. J. Manuf. Process. 57, 787–797 (2020). https://doi.org/10.1016/j.jmapro.2020.07.045
Mo, T.; Chen, J.; Bai, W.; Wu, Y.; Zhang, P.; Zheng, B.: Ni / TiC composite electrodeposition on the surface of Ni-based superalloy. Surf. Coat. Technol. (2021). https://doi.org/10.1016/j.surfcoat.2021.127611
Pompei, E.; Magagnin, L.; Lecis, N.; Cavallotti, P.L.: Electrodeposition of nickel – BN composite coatings. Electrochim. Acta. 54, 2571–2574 (2009). https://doi.org/10.1016/j.electacta.2008.06.034
Chattopadhyay, A.K.; Mohanavel, V.; Ravichandran, M.: Electrodeposition of Ni-Nitride composite coatings : A review of recent study Electrodeposition of Ni-Nitride composite coatings : A review of recent study. IOP Conf. Ser. Mater. Sci. Eng. Pap. (2021). https://doi.org/10.1088/1757-899X/1098/6/062053
Lelevic, A.; Walsh, F.C.: Electrodeposition of Ni e P alloy coatings : A review. Surf. Coat. Technol. 369, 198–220 (2019). https://doi.org/10.1016/j.surfcoat.2019.03.055
Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S.: Electrodeposition of Ni-Fe alloys, composites, and nano coatings e A review. J. Alloys Compd. 691, 841–859 (2017). https://doi.org/10.1016/j.jallcom.2016.08.329
Zakeri, A.; Balashadehi, M.M.; Sabour, A.; Aghdam, R.: Development of hybrid electrodeposition/slurrf diffusion aluminide coatings on Ni-based superalloy with enhanced hot corrosion resistance. J. Compos. Compd. 3, 1–8 (2021)
Li, S.; Song, G.; Zhang, Y.; Fu, Q.; Pan, C.: Graphene-Reinforced Zn − Ni Alloy Composite Coating on Iron Substrates by Pulsed Reverse Electrodeposition and Its High Corrosion Resistance. ACS Omega (2021). https://doi.org/10.1021/acsomega.1c00977
Zhang, W.; Du, S.; Li, B.; Mei, T.; Miao, Y.: Synthesis and characterization of TiN nanoparticle reinforced binary Ni-Co alloy coatings. J. Alloys Compd. 865, 158722 (2021). https://doi.org/10.1016/j.jallcom.2021.158722
Gómez, E.; Pané, S.; Vallés, E.: Electrodeposition of Co-Ni and Co-Ni-Cu systems in sulphate-citrate medium. Electrochim. Acta. 51, 146–153 (2005). https://doi.org/10.1016/j.electacta.2005.04.010
Green, T.A.; Russell, A.E.; Roy, S.: The development of a stable citrate electrolyte for the electrodeposition of copper-nickel alloys. J. Electrochem. Soc. 145, 875–881 (1998). https://doi.org/10.1149/1.1838360
Kobayashi, T.; Shohji, I.: Joining process of dissimilar materials using three- dimensional electrodeposited Ni-Cu film. Mater. Manuf. Process. 36, 1076–1083 (2021). https://doi.org/10.1080/10426914.2021.1885708
Ngamlerdpokin, K.; Tantavichet, N.: Electrodeposition of nickel e copper alloys to use as a cathode for hydrogen evolution in an alkaline media. Int. J. Hydrogen Energy. 39, 2505–2515 (2013). https://doi.org/10.1016/j.ijhydene.2013.12.013
Pingale, A.D.; Owhal, A.; Katarkar, A.S.; Belgamwar, S.U.; Rathore, J.S.: Recent researches on Cu-Ni alloy matrix composites through electrodeposition and powder metallurgy methods : A review. Mater. Today Proc. (2021). https://doi.org/10.1016/j.matpr.2021.07.145
Deo, Y.; Guha, S.; Sarkar, K.; Mohanta, P.; Pradhan, D.: Applied surface science electrodeposited Ni-Cu alloy coatings on mild steel for enhanced corrosion properties. Appl. Surf. Sci. 515, 146078 (2020). https://doi.org/10.1016/j.apsusc.2020.146078
Goranova, D.; Rashkov, R.; Avdeev, G.; Tonchev, V.: Electrodeposition of Ni – Cu alloys at high current densities : details of the elements distribution. J. Mater. Sci. 51, 8663–8673 (2016). https://doi.org/10.1007/s10853-016-0126-y
Heragh, M.F.; Eskandarinezhad, S.; Dehghan, A.: Journal of Composites and Compounds. 2, 123–128 (2020)
Alizadeh, M.; Safaei, H.: Applied Surface Science Characterization of Ni-Cu matrix, Al 2 O 3 reinforced nano-composite coatings prepared by electrodeposition. Appl. Surf. Sci. 456, 195–203 (2018). https://doi.org/10.1016/j.apsusc.2018.06.095
Li, B.; Mei, T.; Li, D.; Du, S.: Ultrasonics - Sonochemistry Ultrasonic-assisted electrodeposition of Ni-Cu / TiN composite coating from sulphate-citrate bath : Structural and electrochemical properties. Ultrason. - Sonochemistry. 58, 104680 (2019). https://doi.org/10.1016/j.ultsonch.2019.104680
Li, B.; Mei, T.; Li, D.; Du, S.; Zhang, W.: Structural and corrosion behavior of Ni-Cu and Ni-Cu / ZrO 2 composite coating electrodeposited from sulphate-citrate bath at low Cu concentration with additives. J. Alloys Compd. 804, 192–201 (2019). https://doi.org/10.1016/j.jallcom.2019.06.381
Safavi, M.S.; Fathi, M.; Mirzazadeh, S.; Ansarian, A.: Perspectives in corrosion-performance of Ni – Cu coatings by adding Y 2 O 3 nanoparticles. Surf. Eng. 37, 226–235 (2021). https://doi.org/10.1080/02670844.2020.1715543
Sharma, V.K.; Kumar, V.; Joshi, R.S.: Investigation of rare earth particulate on tribological and mechanical properties of Al-6061 alloy composites for aerospace application. Integr. Med. Res. 8, 3504–3516 (2019). https://doi.org/10.1016/j.jmrt.2019.06.025
Vipin, S.; Sharma, K.: A review of recent research on rare earth particulate composite materials and structures with their applications. Trans. Indian Inst. Met. (2021). https://doi.org/10.1007/s12666-021-02338-y
Xue, Y.; Jia, X.; Zhou, Y.; Ma, W.; Li, J.: Tribological performance of Ni – CeO 2 composite coatings by electrodeposition. Surf. Coat. Technol. 200, 5677–5681 (2006). https://doi.org/10.1016/j.surfcoat.2005.08.002
Zeng, Y.B.; Qu, N.S.; Hu, X.Y.: Preparation and Characterization of Electrodeposited Ni-CeO 2 Nanocomposite Coatings with High Current Density. Int. J. Electrochem. Sci. 9, 8145–8154 (2014)
Shanmugasamy, S.; Balakrishnan, K.; Subasri, A.; Ramalingam, S.; Subramania, A.: Development of CeO 2 nanorods reinforced electrodeposited nickel nanocomposite coating and its tribological and corrosion resistance. J. Rare Earths. 36, 1319–1325 (2018). https://doi.org/10.1016/j.jre.2018.06.004
Qu, N.S.; Qian, W.H.; Hu, X.Y.; Zhu, Z.W.: Fabrication of Ni-CeO 2 nanocomposite coatings synthesised via a modified sediment Co-deposition process. Int. J. Electrochem. Sci. 8, 11564–11577 (2013)
Guo, L.; Searson, P.C.: Electrochimica Acta On the influence of the nucleation overpotential on island growth in electrodeposition. Electrochim. Acta. 55, 4086–4091 (2010). https://doi.org/10.1016/j.electacta.2010.02.038
Nelson, B.: Mechanical Properties and Corrosion Behaviour of Nanostructured Cu-rich Cu-Ni Electrodeposited Films. Int. J. Electrochem. Sci. 7, 1288–1302 (2012)
Toloei, A.S.; Stoilov, V.; Northwood, D.O.: The effect of different surface topographies on the corrosion behaviour of nickel. Mater. Charact. VI. 77, 193–204 (2013). https://doi.org/10.2495/MC130171
Hamid, Z.A.: Performance of Ni – Cu – ZrO 2 nanocomposite coatings fabricated by electrodeposition technique. Anti-Corrosion Methods Mater. (2017). https://doi.org/10.1108/ACMM-05-2016-1672
Taherimanesh, A.; Rashidi, A.M.: The Effect of Bath pH and Temperature on the Corrosion Behavior of Co-Electrodeposited Ni-Cu / Cr 2 O 3 Nanocomposite Coatings. J. Mater. Eng. Perform. 29, 7863–7871 (2020). https://doi.org/10.1007/s11665-020-05301-y
Lvovich, V.F.: Impedance Spectroscopy Applications to Electrochemical and Dielectric Phenomena, John Wiley & Sons. (2012)
Scully, J.R.; Silverman, D.C.; Kendig, M.: Electrochemical Impedance: Analysis and Interpretation, ASTM STP 1188. Philadelphia: American Society for Testing and Materials. (1993)
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The author declares that there is no conflict of interest regarding the publication of this article.
Rights and permissions
About this article
Cite this article
Unveroglu, B. Electrodeposition and Characterization of Ni-Cu Alloy and Submicron-Sized CeO2 Reinforced Ni-Cu Metal Matrix Composite Coatings. Arab J Sci Eng 48, 145–157 (2023). https://doi.org/10.1007/s13369-022-06783-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-06783-9