Abstract
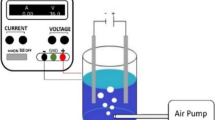
This study was aimed to develop an electrochemical electrode for the degradation of chemical oxygen demand (COD) of the textile industrial wastewater using a full factorial experimental design. Electrochemical method is noble, effective, and efficient in solving the current environmental pollution challenge. Characterization of the textile industrial wastewater was carried out as per the standard method of APHA. The DC power supply of 15.4 V was used to operate the electrochemical treatment system whereas certain chemicals such as sulphuric acid 1.5 mg/L, nitric acid 6.3 M, and oxalic acid solution 100 g/L were used for surface modification of electrodes. Three factors with three-level such as the reaction time of 12, 16, and 20 min, pH, 4, 6, and 7, and NaCl electrolyte concentrations of 2, 4, and 6 g/L were used. The experimental design of the study was formulated as a 33 which was expected to generate 27 runs but the number of experimental runs was reduced to 20 runs using Design Expert 12. The BOD5, COD, and pH of the textile industrial effluent were found to be 430.00 ± 3.00 mg/L, 1730.00 ± 2.00 mg/L, and pH 6.88 ± 0.20, respectively. The maximum COD removal of 94.1% was recorded at the optimum experimental condition of reaction time 16 min, pH 4, and electrolyte concentration 6 g/L whereas the minimum COD removal value of 65.9% was obtained. The regression analysis of COD removal (R2 0.98) indicated that electrolyte concentration was the dominant factor for the degradation of electrochemical. Generally, electrochemical degradation is a promising treatment technology to be implemented to remediate textile industrial wastewater.






Similar content being viewed by others
Data Availability
All data are fully available without restriction.
References
Yang, K.; Liu, Y.; Li, Y.; Cao, Z.; Zhou, C.; Wang, Z.; Zhou, X.; Ali, S.; Xu, X.: Applications and characteristics of Fe-Mn binary oxides for Sb (V) removal in textile wastewater : selective adsorption and the fixed-bed column study. Chemosphere 232, 254–263 (2019). https://doi.org/10.1016/j.chemosphere.2019.05.194
Paździor, K.; Bilińska, L.; Ledakowicz, S.: A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 376, 120597 (2019). https://doi.org/10.1016/j.cej.2018.12.057
Yaseen, D.A.; Scholz, M.: Textile dye wastewater characteristics and constituents of synthetic effluents : a critical review. Springer, Berlin Heidelberg (2018)
Hua, Y.; Xiao, J.; Zhang, Q.; Cui, C.; Wang, C.: Facile synthesis of surface-functionalized magnetic nanocomposites for effectively selective adsorption of cationic dyes. Nanoscale Res. Lett. (2018). https://doi.org/10.1186/s11671-018-2476-7
Azzaz, A.A.; Bengharez, S.J.Z.; Akrout, L.B.H.: Investigations on a dye desorption from modified biomass by using a low-cost eluent: hysteresis and mechanisms exploration. Int. J. Environ. Sci. Technol. 16, 7393–7408 (2019). https://doi.org/10.1007/s13762-018-2171-3
Ma, J.; Jia, Y.; Jing, Y.; Yao, Y.; Sun, J.: Kinetics and thermodynamics of methylene blue adsorption by cobalt-hectorite composite. Dye. Pigment 93, 1441–1446 (2012). https://doi.org/10.1016/j.dyepig.2011.08.010
Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A.: Adsorption of methylene blue on low-cost adsorbents: a review. J. Hazard Mater. 177, 70–80 (2010). https://doi.org/10.1016/j.jhazmat.2009.12.047
Liyana, A.; Hanis, N.; Hairom, H.; Yong, L.; Yin, C.; Khairul, M.; Wahab, A.: Industrial textile wastewater treatment via membrane photocatalytic reactor (MPR) in the presence of ZnO-PEG nanoparticles and tight ultra fi ltration. J. Water Process Eng. 31, 100872 (2019). https://doi.org/10.1016/j.jwpe.2019.100872
Sivarajasekar, N.; Baskar, R.: Adsorption of basic red 9 on activated waste Gossypium hirsutum seeds: process modeling, analysis and optimization using statistical design. J. Ind. Eng. Chem. 20, 2699–2709 (2014). https://doi.org/10.1016/j.jiec.2013.10.058
Adegoke, K.A.; Solomon, O.: Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 12, 8–24 (2015). https://doi.org/10.1016/j.wri.2015.09.002
UN-Water: Water for a sustainable world, The United Nations World Water Development Report 2015 Report WATER. Paris, (2015)
Mekonnen, M.M.; Hoekstra, Y.A.: Four billion people facing severe water scarcity. Am. Assoc. Adv. Sci. 2, 1–7 (2016). https://doi.org/10.1016/j.acra.2014.09.014
Pintilie, L.; Torres, C.M.; Teodosiu, C.; Castells, F.: Urban wastewater reclamation for industrial reuse: An LCA case study. J. Clean. Prod. 139, 1–14 (2016). https://doi.org/10.1016/j.jclepro.2016.07.209
Blus, K.; Foszpa, M.; Gmurek, M.; Bili, L.: Catalytic ozonation of textile wastewater as a polishing step after industrial scale electrocoagulation. J. Environ. Manag. (2020). https://doi.org/10.1016/j.jenvman.2020.110502
Pathak, A.K.; Kothari, R.; Tyagi, V.V.; Anand, S.: Integrated approach for textile industry wastewater for efficient hydrogen production and treatment through solar PV electrolysis. Int. J. Hydrog. Energy. 45, 25768–25782 (2020). https://doi.org/10.1016/j.ijhydene.2020.03.079
Mahdi, M.; Shirazi, A.; Bazgir, S.; Meshkani, F.: A novel dual-layer, gas-assisted electrospun, nano fi brous SAN4-HIPS membrane for industrial textile wastewater treatment by direct contact membrane distillation (DCMD). J. Water Process Eng. 36, 101315 (2020). https://doi.org/10.1016/j.jwpe.2020.101315
Sen, S.K.; Patra, P.; Das, C.R.; Raut, S.; Raut, S.: Pilot-scale evaluation of bio-decolorization and biodegradation of reactive textile wastewater: an impact on its use in irrigation of wheat crop. Water Resour. Ind. 21, 100106 (2019). https://doi.org/10.1016/j.wri.2019.100106
Bouazizi, A.; Saja, S.; Achiou, B.; Ouammou, M.; Calvo, J.I.; Aaddane, A.; Younssi, S.A.: Applied clay science elaboration and characterization of a new fl at ceramic MF membrane made from natural moroccan bentonite: application to treatment of industrial wastewater. Appl. Clay Sci. (2016). https://doi.org/10.1016/j.clay.2016.05.009
Yaseen, D.A.; Scholz, M.: Textile dye wastewater characteristics and constituents of synthetic effluents : a critical review. Springer, Berlin Heidelberg (2019)
Kaur, P.; Kushwaha, J.P.; Sangal, V.K.: Transformation products and degradation pathway of textile industry wastewater pollutants in Electro-Fenton process. Chemosphere 207, 690–698 (2018). https://doi.org/10.1016/j.chemosphere.2018.05.114
Lidiya, M.; Gopalakrishnan, A.; Aravindakumar, C.T.: Low-cost multilayered green fiber for the treatment of textile industry waste water. J. Hazard. Mater. 365, 297–305 (2019). https://doi.org/10.1016/j.jhazmat.2018.11.014
Núñez, J.; Yeber, M.; Cisternas, N.; Thibaut, R.; Medina, P.; Carrasco, C.: Application of electrocoagulation for the efficient pollutants removal to reuse the treated wastewater in the dyeing process of the textile industry. J. Hazard. Mater. 371, 705–711 (2019). https://doi.org/10.1016/j.jhazmat.2019.03.030
Fito, J.; Hulle, S.W.H.V.: Wastewater reclamation and reuse potentials in agriculture : towards environmental sustainability. Environ. Dev. Sustain. (2020). https://doi.org/10.1007/s10668-020-00732-y
Tebeje, A.; Worku, Z.; Nkambule, T.T.I.; Fito, J.: Adsorption of chemical oxygen demand from textile industrial wastewater through locally prepared bentonite adsorbent. Int. J. Environ. Sci. Technol. (2021). https://doi.org/10.1007/s13762-021-03230-4
Ayanpeju, K.; Giwa, A.A.; Motunrayo, J.: Optimization studies for decolourization of textile wastewater using a sawdust-based adsorbent. Chem. Data Collect. 27, 100400 (2020). https://doi.org/10.1016/j.cdc.2020.100400
Fito, J.; Abrham, S.; Angassa, K.: Adsorption of methylene blue from textile industrial wastewater onto activated carbon of parthenium hysterophorus. Int. J. Environ. Res. (2020). https://doi.org/10.1007/s41742-020-00273-2
Khadijah, S.; Ha, M.; Othman, D.; Sheng, Z.; Riduan, M.; Kamilah, N.; Ahmad, A.; Rahman, M.A.; Jaafar, J.; Hamimah, S.; Abdul, S.; Harun, Z.: Novel hydroxyapatite-based bio-ceramic hollow fi ber membrane derived from waste cow bone for textile wastewater treatment. Chem. Eng. J. (2020). https://doi.org/10.1016/j.cej.2019.122396
Fito, J.; Said, H.; Feleke, S.; Worku, A.: Fluoride removal from aqueous solution onto activated carbon of Catha edulis through the adsorption treatment technology. Environ. Syst. Res. 8, 1–10 (2019). https://doi.org/10.1186/s40068-019-0153-1
La, R.; Gzara, L.; Hadj, R.; Ha, A.: Treatment of textile wastewater by a hybrid ultra fi ltration / electrodialysis process. Chem. Eng. Process. Process Intensif. 132, 105–113 (2018). https://doi.org/10.1016/j.cep.2018.08.010
Bedada, D.; Angassa, K.; Tiruneh, A.; Kloos, H.; Fito, J.: Chromium removal from tannery wastewater through activated carbon produced from Parthenium hysterophorus weed. Energy Ecol. Environ. 5, 184–195 (2020). https://doi.org/10.1007/s40974-020-00160-8
Salazar, R.; Gallardo-arriaza, J.; Vidal, J.; Rivera-vera, C.; Toledo-neira, C.; Sandoval, M.A.; Cornejo-ponce, L.; Thiam, A.: Treatment of industrial textile wastewater by the solar photoelectro-Fenton process: in fl uence of solar radiation and applied current. Sol. Energy. 190, 82–91 (2019). https://doi.org/10.1016/j.solener.2019.07.072
Huang, Z.; Li, Y.; Chen, W.; Shi, J.; Zhang, N.; Wang, X.; Li, Z.; Gao, L.; Zhang, Y.: Modified bentonite adsorption of organic pollutants of dye wastewater. Mater. Chem. Phys. 202, 266–276 (2017). https://doi.org/10.1016/j.matchemphys.2017.09.028
Singh, K.; Lataye, D.H.; Wasewar, K.L.: Removal of fluoride from aqueous solution by using bael (Aegle marmelos) shell activated carbon: kinetic, equilibrium and thermodynamic study. J. Fluor. Chem. 194, 23–32 (2017). https://doi.org/10.1016/j.jfluchem.2016.12.009
Samarghandi, M.R.; Dargahi, A.; Shabanloo, A.; Nasab, H.Z.; Vaziri, Y.; Ansari, A.: Electrochemical degradation of methylene blue dye using a graphite doped PbO2 anode: optimization of operational parameters, degradation pathway and improving the biodegradability of textile wastewater. Arab. J. Chem. 13, 6847–6864 (2020). https://doi.org/10.1016/j.arabjc.2020.06.038
Zou, R.; Angelidaki, I.; Jin, B.; Zhang, Y.: Feasibility and applicability of the scaling-up of bio-electro-Fenton system for textile wastewater treatment. Environ. Int. 134, 105352 (2020). https://doi.org/10.1016/j.envint.2019.105352
Kamaraj, R.; Vasudevan, S.: Facile one-pot electrosynthesis of Al(OH)3-kinetics and equilibrium modeling for adsorption of 2,4,5-trichlorophenoxyacetic acid from aqueous solution. New J. Chem. 40, 2249–2258 (2016). https://doi.org/10.1039/c5nj02407b
Vasudevan, S.; Kamaraj, R.; Pandiarajan, A.; Vasudevan, S.: Facile one-pot electrosynthesis of zinc hydroxide for the adsorption of hazardous 2-(2-methyl-4-chlorophenoxy) propionic acid (MCPP) from water and its modelling studies. J. Environ. Chem. Eng. 6, 2017–2026 (2018). https://doi.org/10.1016/j.jece.2018.03.011
Pandiarajan, A.; Kamaraj, R.; Vasudevan, S.: Enhanced removal of cephalosporin based antibiotics (CBA) from water by one-pot electrosynthesized Mg(OH)2: a combined theoretical and experimental study to pilot scale. New J. Chem. 41, 4518–4530 (2017). https://doi.org/10.1039/c6nj04075f
Hegazy, A.K.; Abdel-Ghani, N.T.; El-Chaghaby, G.A.: Adsorption of phenol onto activated carbon from Rhazya stricta: determination of the optimal experimental parameters using factorial design. Appl. Water Sci. 4, 273–281 (2014). https://doi.org/10.1007/s13201-013-0143-9
Tayebee, R.; Mazruy, V.: ARTICLE ORIGINAL acid-thermal activated nanobentonite as an economic industrial adsorbent for malachite green from aqueous solutions: optimization, isotherm and thermodynamic studies. J. Water Environ. Nanotechnol. 3, 40–50 (2018). https://doi.org/10.22090/jwent.2018.01.004
APHA: Standard methods for the examination of water and wastewater. American Public Health Association; American Water Works Association; Water Environment Federation, Washington, DC (1998)
Acknowledgements
We would like to thank the Ethiopian Road Authority (ERA) for the research fund and Addis Ababa Science and Technology University (AASTU) for the research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Yehuala, G., Worku, Z., Angassa, K. et al. Electrochemical Degradation of Chemical Oxygen Demand in the Textile Industrial Wastewater Through the Modified Electrodes. Arab J Sci Eng 47, 5911–5922 (2022). https://doi.org/10.1007/s13369-021-05776-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-05776-4