Abstract
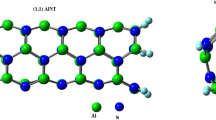
The main challenge war focused on the detection of dopamine drug using a type of AlN nanotube sensor through density functional theory (DFT) calculations. The interaction behavior of dopamine (DA) and aluminum nitride single-wall nanotube (AlNNT) was investigated by using DFT calculation. For the most stable complex, the energy of adsorption of DA on the aluminum nitride single-wall nanotube’s surface was − 17.31 kcal/mol. DA adsorbs through an electrostatic mechanism on the AlNNT. The electrical conductivity of the nanotube has improved significantly by around 28.78 percent through the DA molecules’ absorption on the surface of nanotube. This increase can be used as a signal to detect a drug. Therefore, after DA drug adsorption, the AlNNT work function is reduced from 4.56 to 3.25 eV. Thus, the AlNNT can be both the electronic and work function-type sensor for DA detection. Compared with the gas phase, AlNNT-DA has more stability in aqueous media according to polarizable continuum model (PCM). Eventually, our results also uncovered that, in the presence of environmental contaminants, the AlNNT can selectively recognize the DA molecule.





Similar content being viewed by others
References
Zhou, C.; Li, S.; Zhu, W.; Pang, H.; Ma, H.: A sensor of a polyoxometalate and Au–Pd alloy for simultaneously detection of dopamine and ascorbic acid. Electrochim. Acta 113, 454–463 (2013)
Liu, G.; Ren, G.; Zhao, L.; Cheng, L.; Wang, C.; Sun, B.: Antibacterial activity and mechanism of bifidocin A against Listeria monocytogenes. Food Control 73, 854–861 (2017)
Jiang, D.; Chen, F.-X.; Zhou, H.; Lu, Y.-Y.; Tan, H.; Yu, S.-J., et al.: Bioenergetic crosstalk between mesenchymal stem cells and various ocular cells through the intercellular trafficking of mitochondria. Theranostics 10, 7260 (2020)
Pan, D.; Xia, X.-X.; Zhou, H.; Jin, S.-Q.; Lu, Y.-Y.; Liu, H., et al.: COCO enhances the efficiency of photoreceptor precursor differentiation in early human embryonic stem cell-derived retinal organoids. Stem Cell Res. Ther. 11, 1–12 (2020)
Bas, S.Z.; Cummins, C.; Selkirk, A.; Borah, D.; Ozmen, M.; Morris, M.A.: A novel electrochemical sensor based on metal ion infiltrated block copolymer thin films for sensitive and selective determination of dopamine. ACS Appl. Nano Mater. 2, 7311–7318 (2019)
Szopa, J.; Wilczyński, G.; Fiehn, O.; Wenczel, A.; Willmitzer, L.: Identification and quantification of catecholamines in potato plants (Solanum tuberosum) by GC–MS. Phytochemistry 58, 315–320 (2001)
Myneni, V.R.; Kanidarapu, N.R.; Vangalapati, M.: Methylene blue adsorption by magnesium oxide nanoparticles immobilized with chitosan (CS-MgONP): response surface methodology, isotherm, kinetics and thermodynamic studies. Iran. J. Chem. Chem. Eng. 39, 29–42 (2020)
Kazemi, M.; Zarandi, M.; Zand Monfared, M.R.: Preparation of permanent red 24 nanoparticle by oil in water microemulsion. Iran. J. Chem. Chem. Eng. 39, 43–49 (2020)
Kalhor, M.; Seyedzade, Z.: One-step synthesis of dicyano imidazoles by (NH4)2Ce(NO3)6/HNO3 promoted oxidative cyclocondensation of an aldehyde and 2,3-diaminomaleonitrile. Iran. J. Chem. Chem. Eng. 39, 1–8 (2020)
Munir, S.; Khan, B.; Abdullah, A.; Khan, S.; Naz, S.; Wali, Q.; Tabbasam, N.: Computational investigations of a novel charge transfer complex for potential application in dye-sensitized solar cells. Iran. J. Chem. Chem. Eng. 39, 19–27 (2020)
Zhang, H.-M.; Ma, L.; Li, J.-L.; Zhang, J.-T.; Liu, M.; Zhao, J.-Y.; Zhao, L.: Numerical simulation of reaction mechanism of ethane pyrolysis to form benzene and styrene. Iran. J. Chem. Chem. Eng. 39, 9–18 (2020)
Zhu, S.; Wang, X.; Zheng, Z.; Zhao, X.-E.; Bai, Y.; Liu, H.: Synchronous measuring of triptolide changes in rat brain and blood and its application to a comparative pharmacokinetic study in normal and Alzheimer’s disease rats. J. Pharm. Biomed. Anal. 185, 113263 (2020)
Zhu, S.; Zheng, Z.; Peng, H.; Sun, J.; Zhao, X.-E.; Liu, H.: Quadruplex stable isotope derivatization strategy for the determination of panaxadiol and panaxatriol in foodstuffs and medicinal materials using ultra high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 1616, 460794 (2020)
Jiang, Q.; Jin, S.; Jiang, Y.; Liao, M.; Feng, R.; Zhang, L., et al.: Alzheimer’s disease variants with the genome-wide significance are significantly enriched in immune pathways and active in immune cells. Mol. Neurobiol. 54, 594–600 (2017)
Zou, Q.; Xing, P.; Wei, L.; Liu, B.: Gene2vec: gene subsequence embedding for prediction of mammalian N6-methyladenosine sites from mRNA. RNA 25, 205–218 (2019)
Pashangpour, M.; Peyghan, A.A.: Adsorption of carbon monoxide on the pristine, B-and Al-doped C 3 N nanosheets. J. Mol. Model. 21, 116 (2015)
Rivelino, R.; Dos Santos, R.B.; de Brito Mota, F.; Gueorguiev, G.K.: Conformational effects on structure, electron states, and Raman scattering properties of linear carbon chains terminated by graphene-like pieces. J. Phys. Chem. C 114, 16367–16372 (2010)
Parandin, F.; Jalilian, J.; Jalilian, J.: Tuning of electronic and optical properties in ZnX (X= O, S, Se and Te) monolayer: hybrid functional calculations. Chem. Rev. Lett. 2, 76–83 (2019)
Yang, D.; Wang, S.; Zhang, Q.; Sellin, P.; Chen, G.: Thermal and electrical transport in multi-walled carbon nanotubes. Phys. Lett. A 329, 207–213 (2004)
Shen, C.L.; Lou, Q.; Zang, J.H.; Liu, K.K.; Qu, S.N.; Dong, L., et al.: Near-infrared chemiluminescent carbon nanodots and their application in reactive oxygen species bioimaging. Adv. Sci. 7, 1903525 (2020)
Xiang, W.; Chang, J.; Qu, R.; Albasher, G.; Wang, Z.; Zhou, D., et al.: Transformation of bromophenols by aqueous chlorination and exploration of main reaction mechanisms. Chemosphere 265, 129112 (2020)
Gleiter, H.: Nanostructured materials: basic concepts and microstructure. Acta Mater. 48, 1–29 (2000)
Wang, Y.-C.; Huang, K.; Lai, X.; Shi, Z.; Liu, J.-B.; Qiu, G.: Radical bromination-induced ipso cyclization–ortho cyclization sequence of N-hydroxylethyl-N-arylpropiolamides. Org. Biomol. Chem. 19, 1940–1944 (2021)
Qi, Y.; Wei, J.; Qu, R.; Al-Basher, G.; Pan, X.; Dar, A.A., et al.: Mixed oxidation of aqueous nonylphenol and triclosan by thermally activated persulfate: Reaction kinetics and formation of co-oligomerization products. Chem. Eng. J. 403, 126396 (2021)
Iijima, S.: Helical microtubules of graphitic carbon. Nature 354, 56–58 (1991)
Wang, Z.; Huang, Z.; Brosnahan, J.T.; Zhang, S.; Guo, Y.; Guo, Y., et al.: Ru/CeO2 catalyst with optimized CeO2 support morphology and surface facets for propane combustion. Environ. Sci. Technol. 53, 5349–5358 (2019)
Yang, X.; Li, Q.; Lu, E.; Wang, Z.; Gong, X.; Yu, Z., et al.: Taming the stability of Pd active phases through a compartmentalizing strategy toward nanostructured catalyst supports. Nat. Commun. 10, 1–9 (2019)
Li, J.; Liao, J.; Essawy, H.; Zhang, J.; Li, T.; Wu, Z., et al.: Preparation and characterization of novel cellular/nonporous foam structures derived from tannin furanic resin. Ind. Crops Prod. 162, 113264 (2021)
Chopra, N.G.; Luyken, R.; Cherrey, K.; Crespi, V.H.; Cohen, M.L.; Louie, S.G., et al.: Boron nitride nanotubes. Science 269, 966–967 (1995)
Tondare, V.; Balasubramanian, C.; Shende, S.; Joag, D.; Godbole, V.; Bhoraskar, S., et al.: Field emission from open ended aluminum nitride nanotubes. Appl. Phys. Lett. 80, 4813–4815 (2002)
Chen, X.; Ma, J.; Hu, Z.; Wu, Q.; Chen, Y.: AlN nanotube: round or faceted? J. Am. Chem. Soc. 127, 7982–7983 (2005)
Wu, Q.; Hu, Z.; Wang, X.; Lu, Y.; Chen, X.; Xu, H., et al.: Synthesis and characterization of faceted hexagonal aluminum nitride nanotubes. J. Am. Chem. Soc. 125, 10176–10177 (2003)
Shojaei, Z.; Iravani, E.; Moosavian, S.M.A.; Torab Mostaedi, M.: Lead adsorption onto surface modified nano titania: kinetic and thermodynamic studies. Iran. J. Chem. Chem. Eng. 39, 105–119 (2020)
Kang, D.; Zhirnov, V.; Sanwald, R.; Hren, J.; Cuomo, J.: Field emission from ultrathin coatings of AlN on Mo emitters. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenomena 19, 50–54 (2001)
Dbik, A.; Bentahar, S.; El Messaoudi, N.; El khomri, M.; Lacherai, A.: Removal of methylene blue from aqueous solution by tunics of the corm of the saffron. Iran. J. Chem. Chem. Eng. 39, 95–104 (2020)
Xu, C.; Xue, L.; Yin, C.; Wang, G.: Formation and photoluminescence properties of AlN nanowires. Physica Status Solidi (a) 198, 329–335 (2003)
Anbazhagan, S.; Thiruvengatam, V.; Kulanthai, K.: Adaptive neuro-fuzzy inference system and artificial neural network modeling for the adsorption of methylene blue by novel adsorbent in a fixed - bed column method. Iran. J. Chem. Chem. Eng. 39, 75–93 (2020)
Haber, J.A.; Gibbons, P.C.; Buhro, W.E.: Morphological control of nanocrystalline aluminum nitride: Aluminum chloride-assisted nanowhisker growth. J. Am. Chem. Soc. 119, 5455–5456 (1997)
Kassaee, M.Z.; Khorshidvand, N.: Study of electronic effects on normal vs. abnormal Tetrazol-5-ylidenes at DFT. Iran. J. Chem. Chem. Eng. 39, 63–74 (2020)
Tang, Y.; Cong, H.; Zhao, Z.; Cheng, H.: Field emission from AlN nanorod array. Appl. Phys. Lett. 86, 153104 (2005)
Issabayeva, G.; Yap, N.J.; Ajeel, M.; Hussin, F.; Aroua, M.K.: Removal of zinc from wastewater through the reduction potential determination and electrodeposition using adsorption-desorption solutions. Iran. J. Chem. Chem. Eng. 39, 121–130 (2020)
Liu, C.; Hu, Z.; Wu, Q.; Wang, X.; Chen, Y.; Lin, W., et al.: Synthesis and field emission properties of aluminum nitride nanocones. Appl. Surf. Sci. 251, 220–224 (2005)
Goh, W.; Patriarche, G.; Bonanno, P.; Gautier, S.; Moudakir, T.; Abid, M., et al.: Structural and optical properties of nanodots, nanowires, and multi-quantum wells of III-nitride grown by MOVPE nano-selective area growth. J. Cryst. Growth 315, 160–163 (2011)
Beheshtian, J.; Peyghan, A.A.; Bagheri, Z.: Selective function of Al12N12 nano-cage towards NO and CO molecules. Comput. Mater. Sci. 62, 71–74 (2012)
Gharibzadeh, F.; Vessally, E.; Edjlali, L.; Es haghi, M.; Mohammadi, R.: A DFT study on sumanene, corannulene and nanosheet as the anodes in Li−ion Batteries. Iran. J. Chem. Chem. Eng. 39, 51–62 (2020)
Kamari, E.; Hajizadeh, A.A.; Kamali, M.R.: Experimental investigation and estimation of light hydrocarbons gas-liquid equilibrium ratio in gas condensate reservoirs through artificial neural networks. Iran. J. Chem. Chem. Eng. 39, 163–172 (2020)
Akbari, A.; Fakhri, H.: Three novel sets of Cs2H[PW4Mo8O40] based on various supports: insight into comparative evaluation in oxidative desulfurization. Iran. J. Chem. Chem. Eng. 39, 149–161 (2020)
Kakanakova-Georgieva, A.; Gueorguiev, G.K.; Yakimova, R.; Janzén, E.: Effect of impurity incorporation on crystallization in AlN sublimation epitaxy. J. Appl. Phys. 96, 5293–5297 (2004)
Kenza, A.; Bensmaili, A.; Bouafia-Chergui, S.; Kadmi, Y.: New activated carbon from wormwood as efficient adsorbent of cationic dye in aqueous solution. Iran. J. Chem. Chem. Eng. 39, 137–148 (2020)
Amooey, A.A.: A new film diffusion controlling kinetic model for adsorption at the solid/solution interface. Iran. J. Chem. Chem. Eng. 39, 131–136 (2020)
Yu, Y.-X.: A dispersion-corrected DFT study on adsorption of battery active materials anthraquinone and its derivatives on monolayer graphene and h-BN. J. Mater. Chem. A 2, 8910–8917 (2014)
Peyghan, A.A.; Rastegar, S.F.; Hadipour, N.L.: DFT study of NH3 adsorption on pristine, Ni-and Si-doped graphynes. Phys. Lett. A 378, 2184–2190 (2014)
Pereira, L.; Dos Santos, L.; Fávero, P.; Martin, A.: RM1 semi empirical and DFT: B3LYP/3-21G theoretical insights on the confocal Raman experimental observations in qualitative water content of the skin dermis of healthy young, healthy elderly and diabetic elderly women’s. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 149, 1009–1019 (2015)
Perdew, J.P.; Wang, Y.: Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 45, 13244 (1992)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98(7), 5648–5652 (1993)
Gholizadeh, R.; Yu, Y.-X.: N2O+ CO reaction over Si-and Se-doped graphenes: an ab initio DFT study. Appl. Surf. Sci. 357, 1187–1195 (2015)
Chigo-Anota, E.; Escobedo-Morales, A.; Hernández-Cocoletzi, H.; y López, J.L.: Nitric oxide adsorption on non-stoichiometric boron nitride fullerene: Structural stability, physicochemistry and drug delivery perspectives. Physica E Low-Dimens. Syst. Nanostruct. 74, 538–543 (2015)
Singla, P.; Riyaz, M.; Singhal, S.; Goel, N.: Theoretical study of adsorption of amino acids on graphene and BN sheet in gas and aqueous phase with empirical DFT dispersion correction. Phys. Chem. Chem. Phys. 18, 5597–5604 (2016)
Hadipour, N.L.; Ahmadi Peyghan, A.; Soleymanabadi, H.: Theoretical study on the Al-doped ZnO nanoclusters for CO chemical sensors. J. Phys. Chem. C 119, 6398–6404 (2015)
Weinhold, F.; Landis, C.R.: Natural bond orbitals and extensions of localized bonding concepts. Chem. Educ. Res. Pract. 2, 91–104 (2001)
Richardson, O.: Electron emission from metals as a function of temperature. Phys. Rev. 23, 153 (1924)
Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J.: Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem. Phys. Lett. 255, 327–335 (1996)
Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M.: Carbon nanotube sensors for gas and organic vapor detection. Nano Lett. 3, 929–933 (2003)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassanpour, A., Zamanfar, M., Ebrahimiasl, S. et al. Dopamine Drug Adsorption on the Aluminum Nitride Single-Wall Nanotube: Ab initio Study. Arab J Sci Eng 47, 477–484 (2022). https://doi.org/10.1007/s13369-021-05678-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-05678-5