Abstract
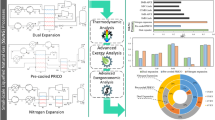
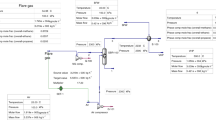
Natural gas flaring is considered one of the significant sources of greenhouse gas emissions in the upstream oil and gas industry. It plays an essential role in reducing the energy efficiency of oil and gas production. Depending on flare gas properties, it can be used for different destinations. Herein, we have evaluated the main flare gas recovery alternatives, including sweet gas production, Gas to Liquid (GTL), and power generation, by using exergy and exergoeconomic analysis tools. For this purpose, flare gas condition in two phases of the Pars Special Economic Energy Zone in south of Iran was chosen as a case study. The mass sensitivity analysis is conducted to examine the processes in different flare gas volumes. Mass flow fluctuation analysis is carried out to analyze the effect of mass flow fluctuation as an intrinsic characteristic of flare systems. Results indicate that the production of electrical power from flaring gas is an economical method regarding the exergoeconomic criteria. While the power generation process has disadvantages such as high exergy destruction and high capital cost, advantages such as higher revenue, local potentials for designing, equipment, and construction should be regarded. On the other hand, the sweetening plant has the highest exergetic efficiency rather than other substitutes. This method also has a lower exergoeconomic factor, especially in lower flaring volume. The mass flow sensitivity analysis in these processes shows the GTL production unit's appropriate performance in low flare gas volume. The results also offer the superior performance of sweetening plants in high flare gas volume. Combined Cycle Power Plant (CCPP) process shows an acceptable performance in a broad range of flare gas volumes. Additionally, mass flow fluctuation analysis has been conducted to determine the effect of mass flow fluctuation in these alternatives. The investigation suggested that the CCPP process has lower fluctuation in terms of cycle product (power generation).












Similar content being viewed by others
Abbreviations
- \(\dot{C}\) :
-
Cost rate ($/h)
- \(\dot{C}_{D}\) :
-
Cost rate of exergy destruction ($/h)
- \(\mathop {{\mathrm{Ex}}}\limits^{ \cdot }\) :
-
Exergy rate (kW)
- \(\dot{m}\) :
-
Mass flow rate (kg/s)
- \(\dot{W}\) :
-
Work rate (kW)
- \(\dot{Z}\) :
-
Investment, operation and maintenance cost rate ($/h)
- c :
-
Cost per exergy unit ($/GJ)
- \(c_{F}\) :
-
Unit cost of the fuel ($/GJ)
- \(c_{P}\) :
-
Unit cost of the product ($/GJ)
- e :
-
Specific exergy (kJ/kg)
- f :
-
Exergoeconomic factor (%)
- h :
-
Specific enthalpy (kJ/kg)
- I :
-
Irreversibility or exergy destruction (kW)
- P :
-
Pressure
- r :
-
Relative cost difference (%)
- s :
-
Specific entropy (kJ/kg-C)
- T :
-
Temperature (°C)
- x :
-
Mole fraction
- Z :
-
Purchased equipment cost
- ε :
-
Exergetic efficiency (%)
- \(\varphi\) :
-
Maintenance factor
- 0:
-
Reference state
- AC:
-
Air cooler
- Air comp:
-
Air compressor
- ATR:
-
Autothermal reactor
- ASF:
-
Anderson–Schulz Flory
- bcm:
-
Billion cubic meters
- C:
-
Compressor
- CC:
-
Combustion chamber
- CCPP:
-
Combined cycle power plant
- CCS:
-
Carbon capture and storage
- ch:
-
Chemical
- CHP:
-
Combined heat and power
- CRF:
-
Capital recovery factor
- D:
-
Destruction
- DEA:
-
Diethanol amine
- DME:
-
Dimethyl ether
- E:
-
Heat exchanger
- F:
-
Fuel
- FGR:
-
Flare gas recovery
- FT:
-
Fischer–Tropsch
- GT:
-
Gas turbine
- GTE:
-
Gas to ethylene
- GTG:
-
Gas turbines generation
- GTL:
-
Gas to liquid
- HRSG:
-
Heat recovery steam generator
- HP:
-
High pressure
- i:
-
Inlet
- k :
-
kTh component
- L:
-
Loss
- LHV:
-
Lower heating value
- LNG:
-
Liquefied natural gas
- Mix:
-
Mixer
- MMSCFD:
-
Million standard cubic feet per day
- o:
-
Outlet
- P:
-
Product
- ph:
-
Physical
- PEC:
-
Purchased equipment cost
- ROR:
-
Rate of return
- ST:
-
Steam turbine
- TEE:
-
Splitter
- V:
-
Vessel
- VLV:
-
Valve
- W:
-
Related to work
References
Elvidge, C.D.; Ziskin, D.; Baugh, K.E.; Tuttle, B.T.; Ghosh, T.; Pack, D.W.; Erwin, E.H.; Zhizhin, M.: A fifteen year record of global natural gas flaring derived from satellite data. Energies 2, 595–622 (2009)
Farina, M.F.: Flare gas reduction—recent global and policy considerations. In: GE Energy, Global Strategy and Planning, (2010)
Zolfaghari, M.; Pirouzfar, V.; Sakhaeinia, H.: Technical characterization and economic evaluation of recovery of flare gas in various gas-processing plants. Energy 124, 481–491 (2017). https://doi.org/10.1016/j.energy.2017.02.084
GROUP, W.B.: Flared Gas Utilization Strategy, Opportunities for Small-Scale Uses of Gas. In: The International Bank for Reconstruction and Development/The World Bank, Washington, (2004)
United Nations, F.C.C.C.: Adoption of the Paris Agreement, Conference of the Parties, Twenty-first session. In: Paris, (30 November to 11 December 2015)
Soltanieh, M.; Zohrabian, A.; Gholipour, M.J.; Kalnay, E.: A review of global gas flaring and venting and impact on the environment: case study of Iran. Int. J. Greenhouse Gas Control 49, 488–509 (2016). https://doi.org/10.1016/j.ijggc.2016.02.010
Davoudi, M.; Rahimpour, M.R.; Jokar, S.M.; Nikbakht, F.; Abbasfard, H.: The major sources of gas flaring and air contamination in the natural gas processing plants: a case study. J. Nat. Gas Sci. Eng. 13, 7–19 (2013). https://doi.org/10.1016/j.jngse.2013.03.002
Hajizadeh, A.; Mohamadi-Baghmolaei, M.; Azin, R.; Osfouri, S.; Heydari, I.: Technical and economic evaluation of flare gas recovery in a giant gas refinery. Chem. Eng. Res. Des. 131, 506–519 (2018). https://doi.org/10.1016/j.cherd.2017.11.026
Bank, T.W.: Flared gas utilization strategy opportunities for smallscale uses of gas. In. The International Bank for Reconstruction and Development, Washington, DC, (2004)
Azin, R.; Asgari, M.; Osfouri, S.; Fatehi, R.; Mehrabi, N.: Geological identification and storage capacity of suitable formation for CO2 disposal in Eastern Zagros (Fars area), Iran. Environ. Stud. Persian Gulf 2(1), 45–55 (2015)
Azin, R.; Mehrabi, N.; Shahriar, O.; Asgari, M.: An overview of CCS road map and identification of a suitable CO2 disposal site in Eastern Zagros (Fars Area) in Iran. Proc. Earth Planet. Sci. 15, 407–412 (2015). https://doi.org/10.1016/j.proeps.2015.08.020
Azin, R.; Malakooti, R.; Helalizadeh, A.; Zirrahi, M.: Investigation of underground sour gas storage in a depleted gas reservoir. Oil Gas Sci. Technol. Rev. IFP Energies nouvelles 69(7), 1227–1236 (2014)
Mourad, D.; Ghazi, O.; Noureddine, B.: Recovery of flared gas through crude oil stabilization by a multi-staged separation with intermediate feeds: a case study. Korean J. Chem. Eng. 26(6), 1706–1716 (2009). https://doi.org/10.1007/s11814-009-0236-1
Beal, C.M.; Davidson, F.T.; Webber, M.E.; Quinn, J.C.: Flare gas recovery for algal protein production. Algal Res. 20, 142–152 (2016). https://doi.org/10.1016/j.algal.2016.09.022
Dong, L.; Wei, S.; Tan, S.; Zhang, H.: GTL or LNG: which is the best way to monetize “stranded” natural gas? Petrol. Sci. 5(4), 388–394 (2008). https://doi.org/10.1007/s12182-008-0063-8
Bachu, S.; Gunter, W.D.: Overview of acid-gas injection operations in Western Canada A2—Wilson, E.S. Rubin, D.W. Keith, C.F. Gilboy, M. In: Thambimuthu, T.M.G. (ed.) Greenhouse Gas Control Technologies 7. pp. 443–448. Elsevier Science Ltd, Oxford (2005)
Zadakbar, O.A.V.A.K.K.: Flare gas recovery in oil and gas refineries. Oil Gas Sci. Technol. 63, 705–711 (2008)
Castelo Branco, D.A.; Szklo, A.S.; Schaeffer, R.: Co2e emissions abatement costs of reducing natural gas flaring in Brazil by investing in offshore GTL plants producing premium diesel. Energy 35(1), 158–167 (2009). https://doi.org/10.1016/j.energy.2009.09.006
Rahimpour, M.R.; Ghorbani, A.; Asiaee, A.; Shariati, A.: Conversion of refinery natural purge gases to liquid hydrocarbons in GTL loop with hydrogen-permselective membranes: an alternative to gas flaring. J. Nat. Gas Sci. Eng. 3(3), 461–475 (2011). https://doi.org/10.1016/j.jngse.2011.04.004
Mohammad, R.; Rahimpour, S.M.J.: Feasibility of flare gas reformation to practical energy in Farashband gas refinery: no gas flaring. J. Hazard. Mater. 209–210, 204–217 (2012)
Rahimpour, M.R.; Jamshidnejad, Z.; Jokar, S.M.; Karimi, G.; Ghorbani, A.; Mohammadi, A.H.: A comparative study of three different methods for flare gas recovery of Asalooye Gas Refinery. J. Nat. Gas Sci. Eng. 4, 17–28 (2012)
Ghorbani, A.; Jafari, M.; Rahimpour, M.R.: A comparative simulation of a novel gas to liquid (GTL) conversion loop as an alternative to a certain refinery gas flare. J. Nat. Gas Sci. Eng. 11, 23–38 (2013). https://doi.org/10.1016/j.jngse.2012.11.002
Chen, L.; Xu, Q.; Gossage, J.L.; Lou, H.H.: Simulation and economic evaluation of a coupled thermal vapor compression desalination process for produced water management. J. Nat. Gas Sci. Eng. 36, 442–453 (2016). https://doi.org/10.1016/j.jngse.2016.10.057
Saidi, M.: Application of catalytic membrane reactor for pure hydrogen production by flare gas recovery as a novel approach. Int. J. Hydrogen Energy 43(31), 14834–14847 (2018). https://doi.org/10.1016/j.ijhydene.2018.05.156
Khanipour, M.; Mirvakili, A.; Bakhtyari, A.; Farniaei, M.; Rahimpour, M.R.: A membrane-assisted hydrogen and carbon oxides separation from flare gas and recovery to a commercial methanol reactor. Int. J. Hydrogen Energy 45(12), 7386–7400 (2020). https://doi.org/10.1016/j.ijhydene.2019.04.149
Group, W.B.: Global Gas Flaring Reduction Partnership (GGFR). In. (2015)
Standards, T.I.P.: ENGINEERING STANDARD FOR PROCESS DESIGN OF GAS TREATING UNITS PART 1—PROCESS DESIGN OF GAS SWEETENING UNITS. In: The Iranian Petroleum Standards, Iran, (2013)
Hall, K.R.: A new gas to liquids (GTL) or gas to ethylene (GTE) technology. Catal. Today 106(1), 243–246 (2005). https://doi.org/10.1016/j.cattod.2005.07.176
Dautzenberg, F.M.; Mukherjee, M.: Process intensification using multifunctional reactors. Chem. Eng. Sci. 56(2), 251–267 (2001). https://doi.org/10.1016/S0009-2509(00)00228-1
Gabriel, K.J.; Noureldin, M.; El-Halwagi, M.M.; Linke, P.; Jiménez-Gutiérrez, A.; Martínez, D.Y.: Gas-to-liquid (GTL) technology: targets for process design and water-energy nexus. Curr. Opin. Chem. Eng. 5, 49–54 (2014). https://doi.org/10.1016/j.coche.2014.05.001
Martínez, D.Y.; Jiménez-Gutiérrez, A.; Linke, P.; Gabriel, K.J.; Noureldin, M.M.B.; El-Halwagi, M.M.: Water and energy issues in gas-to-liquid processes: assessment and integration of different gas-reforming alternatives. ACS Sustain. Chem. Eng. 2(2), 216–225 (2014). https://doi.org/10.1021/sc4002643
Gabriel, K.J.; Linke, P.; Jiménez-Gutiérrez, A.; Martínez, D.Y.; Noureldin, M.; El-Halwagi, M.M.: Targeting of the water-energy nexus in gas-to-liquid processes: a comparison of syngas technologies. Ind. Eng. Chem. Res. 53(17), 7087–7102 (2014). https://doi.org/10.1021/ie4042998
Rajabi, M.; Mehrpooya, M.; Haibo, Z.; Huang, Z.: Chemical looping technology in CHP (combined heat and power) and CCHP (combined cooling heating and power) systems: a critical review. Appl. Energy 253, 113544 (2019). https://doi.org/10.1016/j.apenergy.2019.113544
Dincer, I.; Zamfirescu, C.: Chapter 5—conventional power generating systems. In: Dincer, I.; Zamfirescu, C. (Eds.) Advanced Power Generation Systems, pp. 199–310. Elsevier, Boston (2014)
Jafarian, H.; Sattari, S.; Lajevardi, H.: Technical and economical investigation of electricity generation from associated gas. IEIJQP 4(1), 33–47 (2015)
Ibrahim, T.K.; Mohammed, M.K.; Awad, O.I.; Abdalla, A.N.; Basrawi, F.; Mohammed, M.N.; Najafi, G.; Mamat, R.: A comprehensive review on the exergy analysis of combined cycle power plants. Renew. Sustain. Energy Rev. 90, 835–850 (2018). https://doi.org/10.1016/j.rser.2018.03.072
Baukal, C.: The John Zink Combustion Handbook. CRC Press, Boca Raton, FL (2001)
Brennan, P.W.F.A.D.: Minimize flaring with flare gas recovery. 81(5), 83–85 (2002).
Allen G.D.W.R.E.; Chan, H.: Flare gas recovery in shell Canada refineries. The Fifth Industrial Energy Technology Conference Houston.
Mokhatab, S.; Poe, W.A.; Mak, J.Y.: Handbook of Natural Gas Transmission and Processing: Principles and Practices: Third Edition. Handbook of Natural Gas Transmission and Processing: Principles and Practices: Third Edition, (2015)
Mitra, S.: A Technical report on gas sweetening by amines chemical engineering and technology 2 (2011).
Linnhoff, B.; Hindmarsh, E.: The pinch design method for heat exchanger networks. Chem. Eng. Sci. 38(5), 745–763 (1983). https://doi.org/10.1016/0009-2509(83)80185-7
Behroozsarand, A.; Zamaniyan, A.: Simulation and optimization of an integrated GTL process. J. Clean. Prod. 142(Part 4), 2315–2327 (2017). https://doi.org/10.1016/j.jclepro.2016.11.045
Kuipers, E.W.; Scheper, C.; Wilson, J.H.; Vinkenburg, I.H.; Oosterbeek, H.: Non-ASF product distributions due to secondary reactions during Fischer-Tropsch synthesis. J. Catal. 158(1), 288–300 (1996). https://doi.org/10.1006/jcat.1996.0028
Van Der Laan, G.P.; Beenackers, A.A.C.M.: Kinetics and selectivity of the Fischer-Tropsch synthesis: a literature review. Catal. Rev. Sci. Eng. 41(3–4), 255–318 (1999)
Venter, J.A.: Modeling and Exergy Analysis of Natural Gas to Hydrocarbon Liquids (GTL) Process. The built environment and information technology, University of Pretoria, Faculty of engineering (2002)
Waste Heat Recovery: Technology and Opportunities in U.S. Industry. U.S. Department of energy U.S.A (2008)
Grosu, L.; Marin, A.; Dobrovicescu, A.; Queiros-Conde, D.: Exergy analysis of a solar combined cycle: organic Rankine cycle and absorption cooling system. Int. J. Energy Environ. Eng. 7(4), 449–459 (2016). https://doi.org/10.1007/s40095-015-0168-y
Tirandazi, B.; Mehrpooya, M.; Vatani, A.; Moosavian, S.M.A.: Exergy analysis of C2+ recovery plants refrigeration cycles. Chem. Eng. Res. Des. 89(6), 676–689 (2011). https://doi.org/10.1016/j.cherd.2010.10.006
Adrian Bejan, G.T.T.S.N.I.; Michael, M.: Thermal Design and Optimization. Wiley (1996)
Michael J.M.H.N.S.; Daisie D.B.; Margaret B.B: Fundamentals of Engineering Thermodynamics. Wiley (2003)
Dedication A2 - Kotas, T.J. In: The Exergy Method of Thermal Plant Analysis. p. ii. Butterworth-Heinemann, (1985)
Truls G.E.: Exergy analysis of conventional and electrified oil and gas platforms. In: (2014)
Dincer, I.; Rosen, M.A.: Exergy 2ed. Elsevier, Oxford, UK (2013)
EM, S.: Advances in thermal design of heat exchangers. Wiley (2005)
Wu, C.; Wang, S.-S.; Feng, X.-J.; Li, J.: Energy, exergy and exergoeconomic analyses of a combined supercritical CO2 recompression Brayton/absorption refrigeration cycle. Energy Convers. Manag. 148, 360–377 (2017). https://doi.org/10.1016/j.enconman.2017.05.042
Ansarinasab, H.; Mehrpooya, M.; Mohammadi, A.: Advanced exergy and exergoeconomic analyses of a hydrogen liquefaction plant equipped with mixed refrigerant system. J. Clean. Prod. 144, 248–259 (2017). https://doi.org/10.1016/j.jclepro.2017.01.014
Panjeshahi, M.H.; Harati, F.; Nouzari, M.: Improving energy efficiency in natural gas refineries using exergy analysis. Chem. Eng. Trans. 21, 121–126 (2010)
Ashrafizadeh, S.A.; Amidpour, M.; Abolmashadi, M.: Exergy analysis of distillation column using concept of driving forces. J. Chem. Eng. Jpn. 46(7), 434–443 (2013). https://doi.org/10.1252/jcej.11we038
Lazzaretto, A.; Tsatsaronis, G.: SPECO: a systematic and general methodology for calculating efficiencies and costs in thermal systems. Energy 31(8–9), 1257–1289 (2006)
Ahmadi, P.I.D.M.A.: Rosen: multi-objective optimization of novel solar-based multi generation energy system. Sol. Energy 108, 576–591 (2014)
Baghernejad, A.; Yaghoubi, M.: Exergoeconomic analysis and optimization of an integrated solar combined cycle system (ISCCS) using genetic algorithm. Energy Convers. Manag. 52(5), 2193–2203 (2011). https://doi.org/10.1016/j.enconman.2010.12.019
Baghernejad, A.; Yaghoubi, M.: Multi-objective exergoeconomic optimization of an integrated solar combined cycle system using evolutionary algorithms. Int. J. Energy Res. 35(7), 601–615 (2011). https://doi.org/10.1002/er.1715
Ghaebi, H.; Amidpour, M.; Karimkashi, S.; Rezayan, O.: Energy, exergy and thermoeconomic analysis of a combined cooling, heating and power (CCHP) system with gas turbine prime mover. Int. J. Energy Res. 35(8), 697–709 (2011). https://doi.org/10.1002/er.1721
Boyaghchi, F.A.; Mahmoodnezhad, M.; Sabeti, V.: Exergoeconomic analysis and optimization of a solar driven dual-evaporator vapor compression-absorption cascade refrigeration system using water/CuO nanofluid. J. Clean. Prod. 139, 970–985 (2016). https://doi.org/10.1016/j.jclepro.2016.08.125
Shokati, N.; Ranjbar, F.; Yari, M.: A comparative analysis of Rankine and absorption power cycles from exergoeconomic viewpoint. Energy Convers. Manag. 88, 657–668 (2014). https://doi.org/10.1016/j.enconman.2014.09.015
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaresharif, M., Vatani, A. & Ghasemian, M. Evaluation of Different Flare Gas Recovery Alternatives with Exergy and Exergoeconomic Analyses. Arab J Sci Eng 47, 5501–5520 (2022). https://doi.org/10.1007/s13369-021-05485-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-05485-y